More Information
Submitted: August 08, 2024 | Approved: August 20, 2024 | Published: August 21, 2024
How to cite this article: Aslam H, Umar A, Khan MU, Honey S, Ullah A, Ashraf MA, et al. A Review on Heavy Metals in Ecosystems, Their Sources, Roles, and Impact on Plant Life. J Genet Med Gene Ther. 2024; 7(1): 020-034. Available from: https://dx.doi.org/10.29328/journal.jgmgt.1001012.
DOI: 10.29328/journal.jgmgt.1001012
Copyright License: © 2024 Aslam H, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Heavy metals (HMs); Plants; Growth and development; HMs toxicity
A Review on Heavy Metals in Ecosystems, Their Sources, Roles, and Impact on Plant Life
Humaira Aslam1, Ali Umar2, Misbah Ullah Khan1*, Shehla Honey1, Aman Ullah2, Muhammad Ahsan Ashraf3, Ghulam Ayesha1, Nazia Nusrat1, M Jamil1, Shahid Khan1 and Adeel Abid1
1Centre for Nanosciences, University of Okara, Okara 56130, Pakistan
2Department of Zoology, Faculty of Life Sciences, University of Okara, Okara 56130, Pakistan
3Department of Zoology, Division of Science and Technology, University of Education, Lahore 54000, Pakistan
*Address for Correspondence: Misbah Ullah Khan, Centre for Nanosciences, University of Okara, Okara 56130, Pakistan, Email: [email protected]
The presence of heavy metals (HMs) on Earth is essential to all forms of life. These metals are essential for plant and animal development but can have numerous negative effects on the living environment. In this review, we looked at where HMs come from, why they are harmful, and how they affect plants. Articles indexed in Google Scholar, PubMed, Research Gate, Science Direct, and a few books on heavy metals were consulted for this study. Heavy metals are essential for plant development and growth. According to this analysis, the hazardous effects of HMs are on the rise all throughout the globe, and this trend may be attributed mostly to human activity. Because of its impact on agricultural productivity and environmental changes, soil pollution caused by HMs is among the most crucial elements. Plants have evolved very sophisticated defense systems to deal with these environmental challenges. The threat that HM stress poses to plants has attracted a lot of attention worldwide because it could stunt agriculture’s long-term expansion. In spite of their importance for plants, this study found that HMs pose a significant threat to plant life. The novelty of this review lies in its detailed examination of both the beneficial and detrimental roles of HMs, providing a balanced perspective often overlooked in current literature. The significance of this work is underscored by its potential to inform sustainable agricultural practices and environmental management strategies, as it highlights the delicate balance required to harness the benefits of HMs while mitigating their risks. Despite their necessity for plant development, this review underscores the significant risks HMs pose to plant health and ecosystems.Less than 10 cases have been reported in the literature of the association of germline BRCA1 and Squamous cell Carcinoma – the esophagus. The article focuses on the probable pathogenesis of BRCA1 mutation with non-classic malignancies and the response of Poly adenosine diphosphate ribose polymerase inhibitors (PARP) inhibitors in such a scenario. We report an unusual manifestation of the BRCA1 gene with second primary oesophageal squamous cell cancer occurring five years later to triple-negative breast cancer.
Heavy metals are metals with atomic masses or densities that are comparatively large. Its definition varies depending on the situation in every field. For instance, a heavy metal may be characterized in terms of its density in metallurgy. Although a chemist would be more interested in a heavy metal’s chemical behavior, physicists can characterize a heavy metal in terms of its atomic number [1].
Heavy metals include the oldest known common elements like copper, iron, and tin as well as pricey elements like silver, platinum, and gold. The first discoveries of gallium, hafnium, and thallium occurred around the turn of the 19th century. Due to the heavy nature of naturally occurring metals like aurum (gold), iron, and copper, as well as their malleability, efforts were made to shape metals into decorations, tools, and weapons throughout prehistory [2].
Leopold Gmelin, a German scientist, divided the elements into non-metals, light metals, and heavy metals in 1817. Heavy metals have a density of 5.308–22 g/cm3, whereas light metals have a density of 0.860–5 g/cm3. Eventually, the phrase began to refer to metals having large atomic masses or numbers. Toxicologist J. Duffus examined the definitions of heavy metals in 2002 and concluded that they were so wildly different that the word was functionally useless. With this finding, the classification of certain metals as heavy was also questioned since they are also light and have a role in biological processes. Despite its ambiguous definition, the phrase “heavy metal” often occurs in scientific literature [3].
Iron, cobalt, zinc, and other vital nutrients are among the heavy metals. Some are safe, like silver and indium, but too much of them may be hazardous. For instance, vanadium pentoxide (V2O5) damages DNA and causes cancer in animals. The liver and kidney are poisoned by the permanganate ion (MnO4-). Heart failure may occur if you consume more iron than 0.5 g. The majority of youngsters who experience this die within 24 hours. At 30 ppm, nickel carbonyl (Ni (CO)4) causes mortality, brain damage, and respiratory failure. The lethal dose of selenium is 5–6 mg ( can have paralytic effects)[4].
Lead, Hg, and cadmium are toxic. Mining, industrial waste, agricultural waste, paints, and treated lumber are key sources of these metals environmentally hazardous heavy metals [5]. Some are poisonous only in excess or in specific forms. Metal fume fever may result from inhaling metal particles or vapors. Due to widespread usage, chromium, arsenic, lead, mercury, and cadmium are harmful. Like mercury compounds, chromium (VI) is hazardous. Sulfur attracts these five elements/heavy metals in the body. They generally bind to metabolic reaction-speeding enzymes [6].
We also need heavy metals. Our bodies contain tiny quantities of these, yet they are essential. Iron was employed extensively. Blood contains it. They create sheets, wires, machines, glass coloring, paints, plastics, and catalysts. Electronics, magnets, lights, and nuclear equipment employ them [7].
The novelty of this review lies in its comprehensive and nuanced examination of the dual nature of Heavy Metals (HMs) in the environment, which is often underrepresented in the existing literature. While most studies tend to focus either on the detrimental effects of heavy metals on plant life or their essential roles in physiological processes, this review brings these two aspects together, providing a balanced perspective that underscores the complexity of HMs’ interactions with plant systems. By integrating an analysis of both the beneficial roles and the toxic effects of HMs, this review sheds light on the intricate mechanisms plants employ to utilize these metals for growth and development while simultaneously defending against their potential toxicity. This dual focus not only enhances our understanding of plant resilience but also highlights gaps in current research, suggesting areas where further study could lead to more refined approaches to managing heavy metal stress in agricultural settings.
The significance of this review extends beyond academic inquiry, offering critical insights with direct implications for sustainable agricultural practices and environmental management. As soil pollution from HMs continues to rise, largely due to anthropogenic activities, the need for informed strategies to mitigate their adverse effects becomes increasingly urgent. This review contributes to this discourse by emphasizing the delicate balance required to harness the benefits of HMs for plant growth while minimizing their toxic impacts. The findings presented here have the potential to inform policy-making and the development of innovative agricultural techniques aimed at enhancing crop resilience in polluted environments. Moreover, the review’s emphasis on the global nature of HM stress underscores the widespread relevance of these issues, making it a valuable resource for researchers, practitioners, and policymakers alike who are working towards ensuring the long-term sustainability of agricultural systems and the preservation of ecological health.
This review systematically examined the literature on the sources, roles, and effects of Heavy Metals (HMs) on plant life, with a focus on publications from 2000 to 2023. The objective was to synthesize existing knowledge and highlight both the beneficial and detrimental impacts of HMs on plants, incorporating studies from various scientific disciplines.
Data collection
The literature search was conducted using multiple academic databases, including Google Scholar, PubMed, ResearchGate, and ScienceDirect. In addition to peer-reviewed journal articles, relevant books, conference papers, and review articles were also included to provide a comprehensive analysis. The time frame for data collection spanned from the year 2000 to 2023 to capture the most relevant and recent developments in the field, while also considering foundational studies that are still influential.
Inclusion criteria
The following criteria were used to determine the inclusion of studies in the review:
1. Relevance: Only studies that directly addressed the role, sources, and effects of HMs on plant life were included. This encompassed research on HM toxicity, soil contamination, plant physiology, and the mechanisms plants use to cope with HM stress.
2. Recency and impact: Articles published between 2000 and 2023 were prioritized, with a particular focus on studies from the last decade (2010-2023) to ensure the incorporation of the latest scientific insights and technologies.
3. Quality and credibility: Peer-reviewed articles, high-impact journals, and authoritative books were selected to ensure the scientific rigor of the review.
4. Geographical scope: Studies from different geographical regions were included to provide a global perspective on the issue, acknowledging that the impact of HMs can vary based on environmental and regional factors.
5. Language: Only studies published in English were included to maintain consistency in the review process.
Exclusion criteria
The review excluded the following:
1. Irrelevant topics: Studies that did not directly relate to the effects of HMs on plant life, such as those focusing solely on human or animal health, were not considered.
2. Non-peer-reviewed sources: Articles from non-peer-reviewed journals, opinion pieces, and non-scientific publications were excluded to maintain academic integrity.
3. Outdated research: Studies published before 2000 were generally excluded unless they were seminal works that provided essential background information or foundational theories relevant to the review.
Data analysis
The selected studies were carefully analyzed to identify recurring themes, significant findings, and emerging trends in HM research related to plant life. Emphasis was placed on understanding the dual role of HMs as both essential nutrients and toxicants, as well as the complex defense mechanisms that plants have developed in response to HM stress. The data were synthesized to present a balanced overview of the current state of knowledge, highlighting critical areas where further research is needed and potential implications for sustainable agricultural practices.
Sources of heavy metals
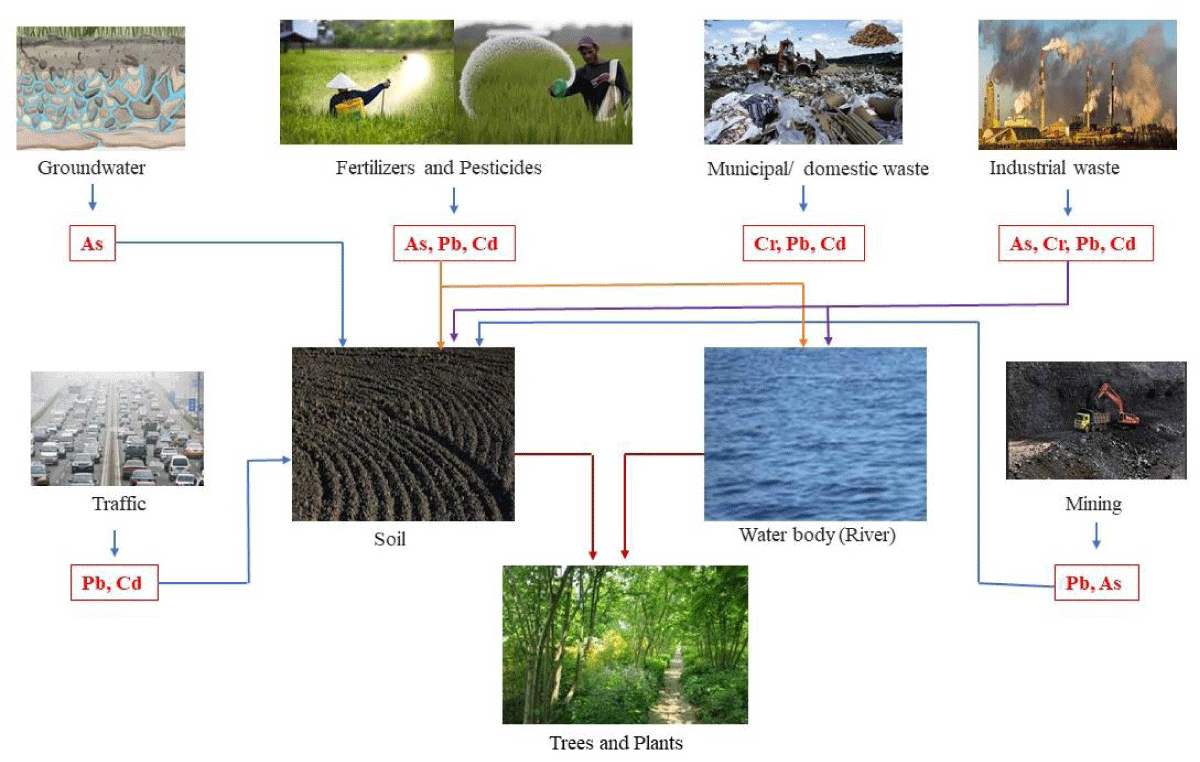
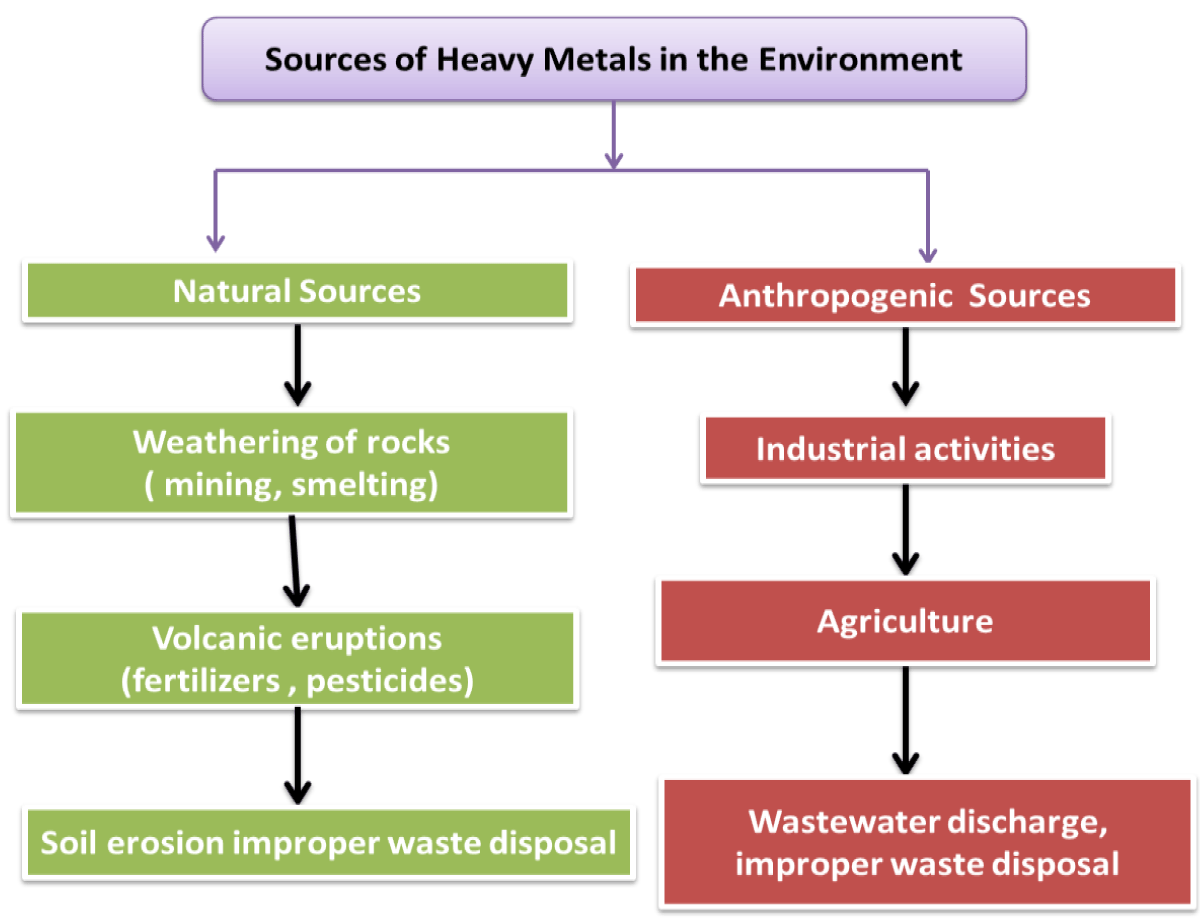
Heavy metals are helpful yet harmful. Heavy metal sources, uses, consequences, mechanism of action, and prevention in plants will be examined. Components with towering nuclear weights and densities at least five times greater than water are dominant metals. They include a variety of metals, including nickel (Ni), arsenic (As), chromium (Cr), lead (Pb), mercury (Hg), and cadmium (Cd). The contradictory nature of heavy metals—that is, their capacity for both support and destruction—may make for an intriguing study topic, given their intricate roles in the mechanical world, the natural world, and human intuition (Figures 1,2).
Figure 1: BrainSources of heavy metals and route to plants.
Figure 2: Schematically Scheme diagram of Sources of Heavy Metals in the Environment.
Origins of the Greatest Metals The common forms and fabricated activities can introduce a significant amount of metals into the ecosystem. Typical sources of metal release into the atmosphere, water, and soil include volcanic eruptions, rock weathering, and soil dissolution. Anthropogenic sources are extremely mechanical processes that release significant amounts of heavy metals into the environment, such as mining, refining, and purifying metals. Furthermore, the use of heavy metals in several industries, such as paint, batteries, hardware, and coloring, adds to their environmental proximity. Agricultural practices that involve phosphate fertilizers and insecticides also introduce heavy metals into the soil and water systems. Wastewater release and the dishonest transfer of mechanical waste exacerbate the degradation [8] (Figure 3).
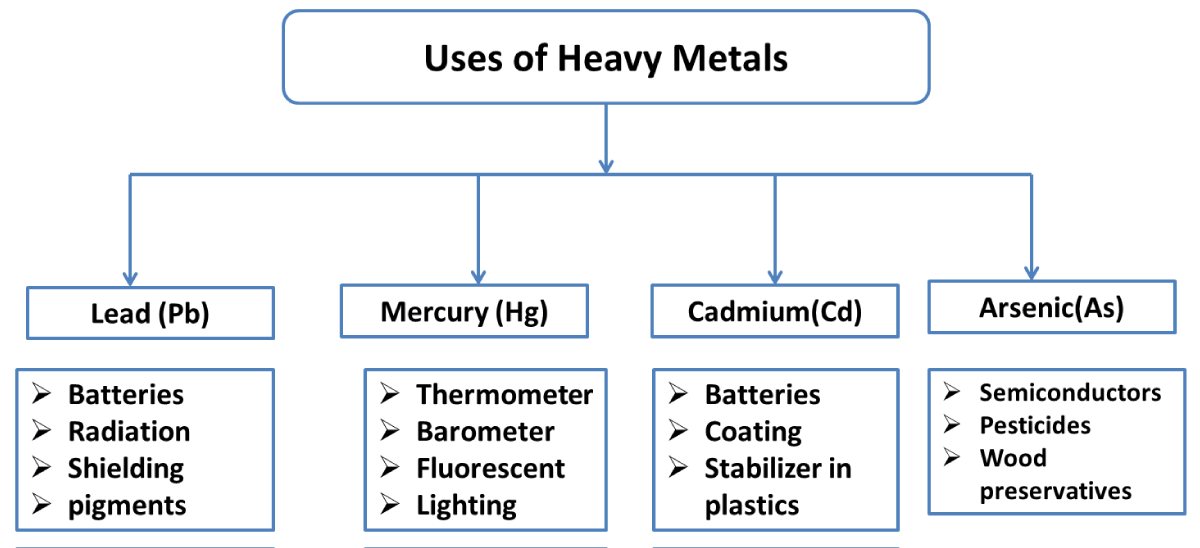
Figure 3: Represent the use of heavy metals.
Lead
Lead’s atomic number is eighty-two (from the Latin plumbum). Dense metal. Lead melts easily. When cut, lead is silvery-blue, but air turns it grey [9]. Lead finishes three heavy element decay chains and has the highest atomic number of any solid material. Prehistoric Western Asians realized lead can be easily mined from its ores. Galena, a lead mineral, often contains silver, which promoted lead mining and usage in ancient Rome. As Rome fell, lead production dropped until the Industrial Revolution. About half of the 10 million tonnes of lead produced globally in 2014 were recycled. Lead is useful because of its density, low melting point, ductility, and oxidation resistance. Due to its low cost and availability, it was used in construction, plumbing, batteries, bullets and shots, weights, solders, pewters, fusible alloys, white paints, leaded gasoline, and radiation shielding[10] (Figure 4).
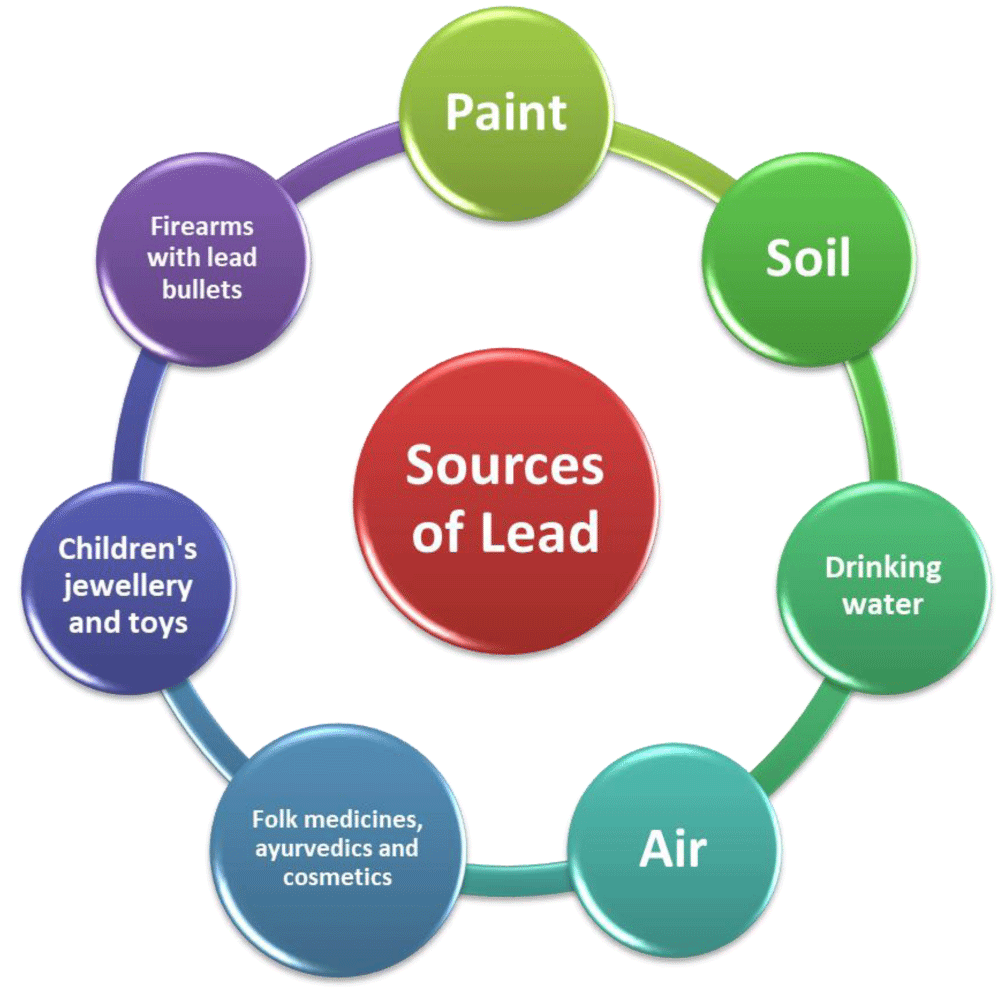
Figure 4: Representation of the sources of lead.
Sources of lead
Inhalation and ingestion are common methods of exposure to lead. In the digestive and respiratory systems, lead is easily absorbed into the body. In addition to routine hand-to-mouth behavior, children’s propensity for discovery increases their risk of lead exposure. A baby is exposed to lead without purposeful ingestion when exposed to lead-covered items, degrading paints, contaminated soil or dust, or lead-containing products. Lead reassessments are often seen in the environment [11]. Before 1978, lead was furthermore a gasoline additive in the USA. Lead solder and pipes may leach into drinking water. Lead from active business or battery disposal may also be discovered in soil. Lead may be found in various places inside and outside your home [12].
Paint
Lead was formerly used in paint to add color, increase coverage, and prolong its lifespan [13]. The use of lead paint in residential construction was made illegal by federal authorities in the year 1978. There is a good chance that paint on the walls of homes built before 1978 contains lead. Toys and furniture that had been painted before 1978 might have also been painted with lead-based paint. Paint that contains lead may become dangerous if it chips, becomes dust, or gets into the soil [14].
Soil
The use of lead in gasoline was made illegal by the federal government in 1996, after having begun a reduction process in 1973. Despite this, lead levels in the environment continue to be elevated due to the combination of vehicle exhaust and dirt from the roadside. Lead levels in soil around busy roadways may also be higher [15]. Metal smelting, battery manufacture, and other lead-using enterprises still produce lead. Lead is released into the air and combines with the soil surrounding homes, particularly if they are close to sources. Outside homes, flaking lead-based paint may mingle with the soil. If homeowners aren’t cautious, lead-based paint and soil may mix. Wind may sweep lead dust into buildings and yards from lead-contaminated soil [16].
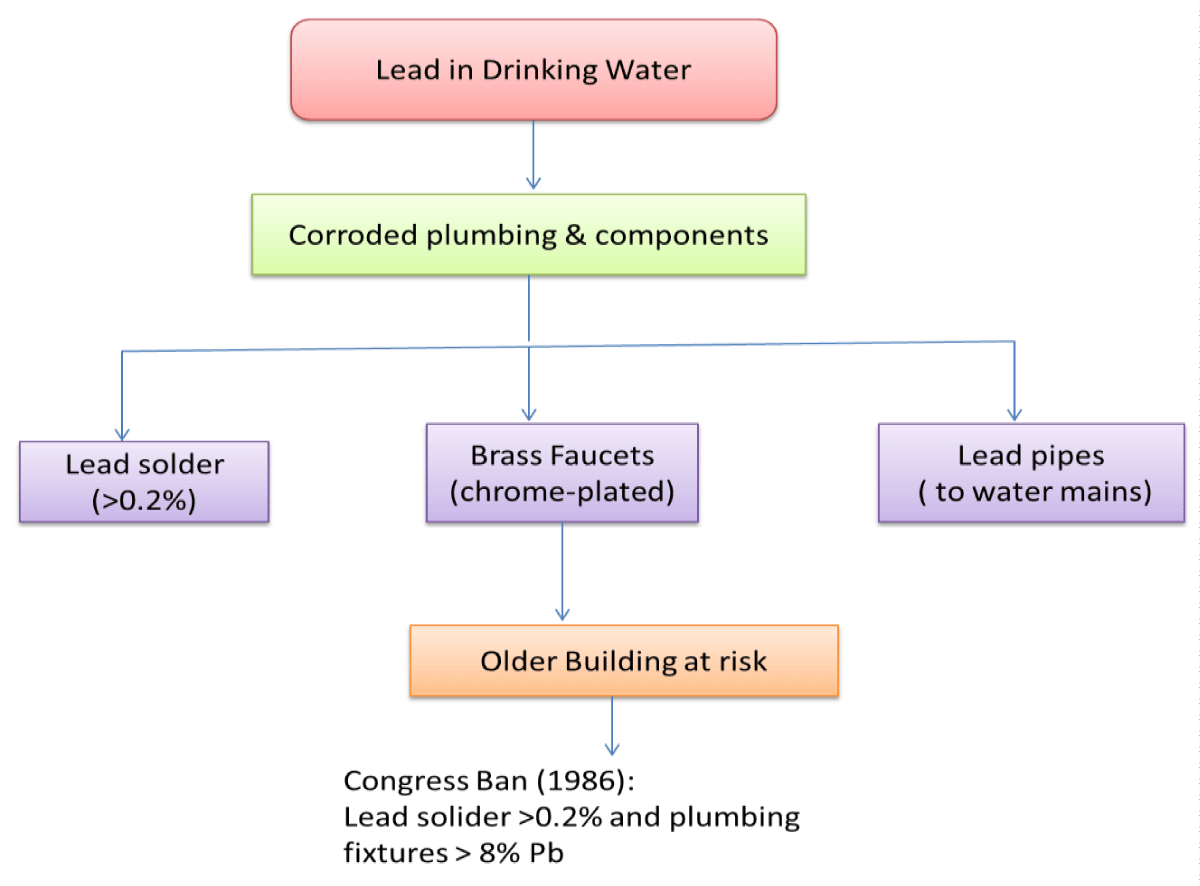
Drinking water
Rivers and lakes seldom have lead. Lead enters drinking water largely via corroded plumbing and lead-containing water distribution system components. [17]. These materials include lead-based solder used to connect copper pipe, brass, and chrome-plated brass faucets, and, in certain cases, lead pipes linking homes and businesses to water mains [18]. Congress banned the use of lead solder containing more than 0.2% lead and set a limit of 8.0% lead for faucets, pipes, and other plumbing fixtures in 1986. It’s possible that plumbing in older buildings is where lead in drinking water comes from [19] (Figure 5).
Figure 5: Representation of Lead Contamination in Drinking Water.
Air
Lead is present in both indoor and outdoor air. Because of industrial sources, lead is mostly present in outdoor air (e.g., smelters, waste incinerators, utilities, and lead-acid battery manufacturers) [20]. Lead from both natural and man-made sources, decaying paint, burning of leaded gasoline and aviation fuel, and deteriorating paint may all be found in wind-blown soil and road dust. Sources of lead in indoor air include suspended dust, outside air, and a variety of hobbies (e.g., making stained glass objects using lead solder, and shooting using lead bullets at indoor firing ranges) [21].
Folk medicines, ayurvedic and cosmetics
Lead is present in folk medicines. They come from Mexico, the Dominican Republic, India, the Middle East, and Southeast Asia. Greta and Azarcon are an example. Rueda, Alarcon, Maria Luisa, and Coral are the names given to Azarcon. Greta, in powdery yellow. For indigestion, they are. Lead is present in pay-loo-ah. Rash and fevers are treated with red powder. Traditional remedies including golf, ghasard, bala, and kandu all contain lead. Cosmetic brands Kohl and Surma are connected [20]. Lead-free Ayurveda is ancient Indian and Asian medicine. Ayurveda remedies include herbs, minerals, metals, or animal products [22].
Children’s jewellery and toys
In the United States, inexpensive children’s jewelry containing lead is sold in vending machines and at large discount stores. It is also seen in low-quality amulets made of metal that are worn for good luck or protection. Moreover, lead may be found in adult costume jewelry. Jewellery is not to be handled by or consumed by children [23].
Firearms with lead bullets
Lead is also found in venison and small game shot with lead bullets. A new study discovered small lead pieces in meat from lead-bullet-shot deer. Some gunshot fragments are too small to see, touch, or chew [24]. On indoor shooting ranges, lead may be discharged when a gun is fired. Lead particles are produced when the bullet spirals through the barrel. Lead dust on food, cigarettes, and other products may be inhaled or ingested [21].
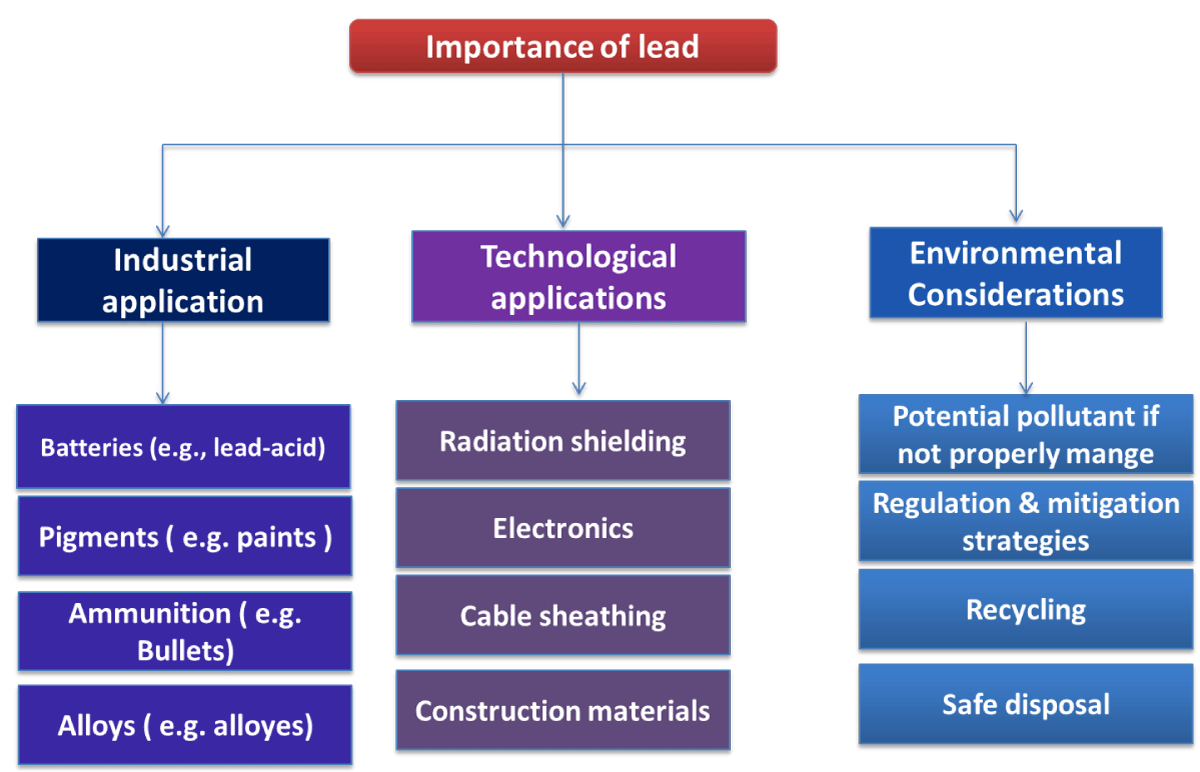
Importance of lead
Due to its high density, lead is employed in nuclear reactors and X-ray equipment as a shield against gamma and X-ray radiation. In certain medical imaging devices, lead is also utilized. In addition to these applications, lead is used in the manufacturing of ammunition, as a material to absorb vibrations and noises, and as a corrosion-resistant coating on some wires and cables [25]. In spite of the fact that lead is prevalent in our surrounding environment, there is no evidence to suggest that it serves any purpose in our bodies. As lead gets into the body, it is confused with calcium and other essential minerals and elements. In the long term, this confusion may be detrimental to the health of both children and adults [26] (Figure 6).
Figure 6: Representation of the importance of lead.
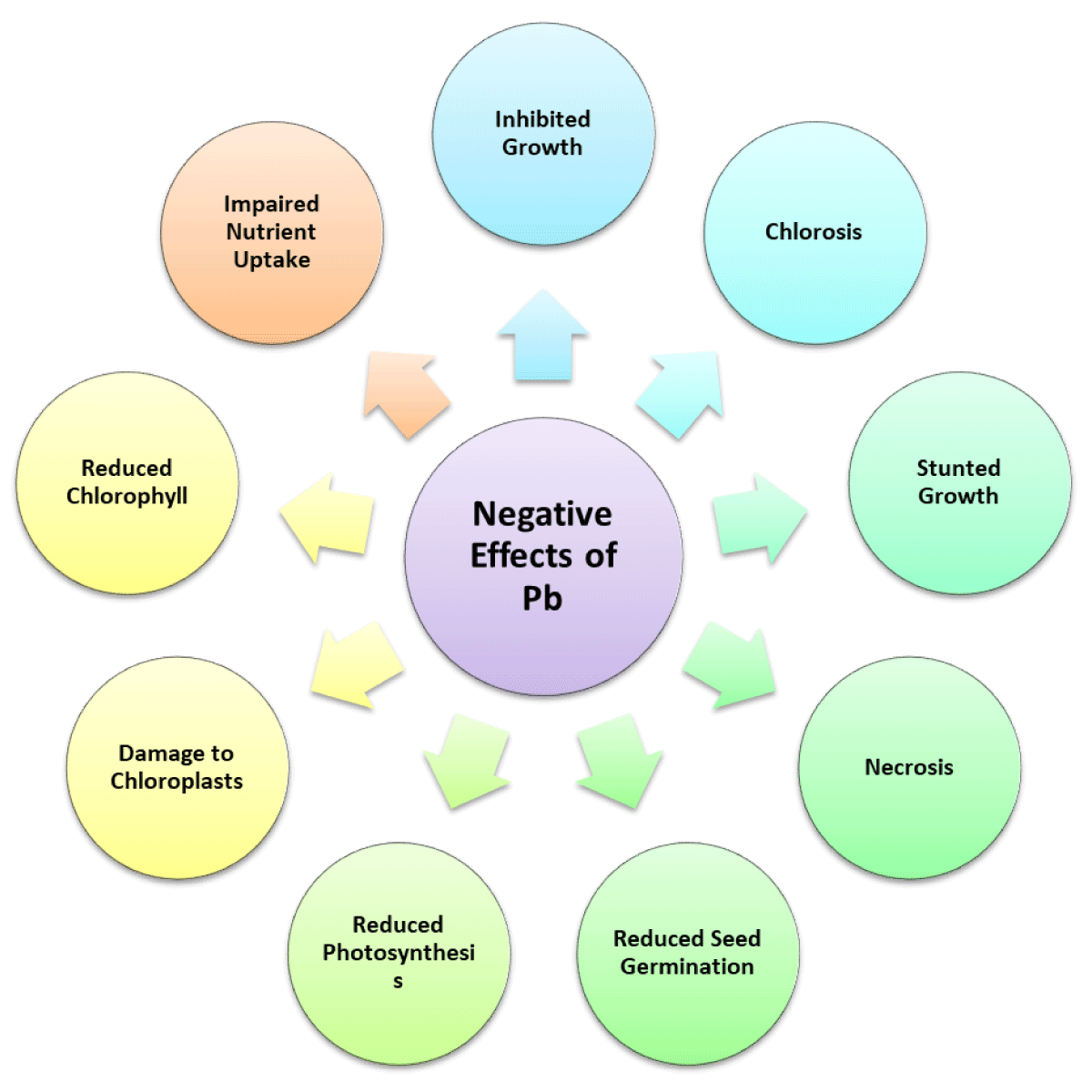
Negative effects of lead on plants
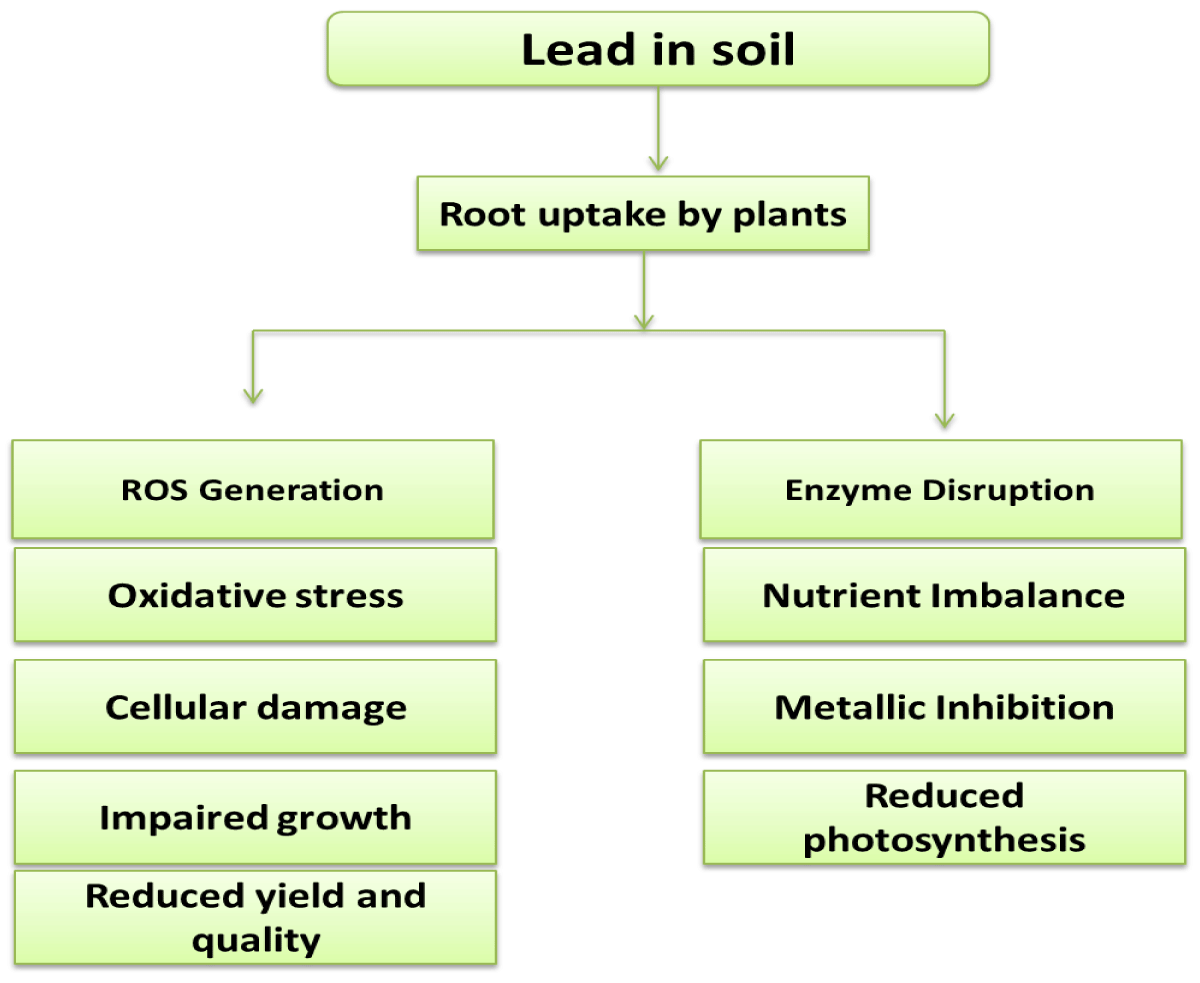
Reactive Oxygen Species (ROS) overproduction from lead poisoning reduces DNA damage, lipid peroxidation, and ATP synthesis. Lead inhibits plant growth, transpiration, chlorophyll production, water content, seed germination, root elongation, seedling development, and protein content. Lead interferes with enzyme activity, mineral nutrition, water balance, and photosynthesis. These ailments impact how plants operate [27].
Soil pollution by lead (Pb) is prevalent. It’s quite toxic. Pb causes morphological, physiological, and biochemical dysfunctions in plants while having no biological role. Pb exposure activates several plant tolerance mechanisms. The majority of Pb is absorbed by roots. Rapidly absorbing huge quantities of Pb and storing it in the vacuole, which is followed by changes in root development and branching pattern, or translocating to the plant’s aboveground sections in the form of hyperaccumulators are all examples of how plant roots react [28].
Rhizosphere Pb levels and root development’s physiological and metabolic processes. Lead poisoning causes rapid root suppression, underdeveloped plant development, root blackening, and chlorosis. Lead hinders photosynthesis, mineral nutrition, water balance, and enzyme function [29]. These abnormalities influence physiological processes in plants. Cells may be killed by high lead doses. Lead also prevents seedling growth and reduces root and shoot dry mass, root and shoot length, tolerance index, and germination percentage. Lead lowered the fresh biomass, growth tolerance index, and root, shoot, and leaf growth of seedlings [30]. Further research found that Pisum sativum and Zea mays grew fewer roots, shoots, and leaves as well as fresh and dry biomass at the predicted lead levels. Lead causes phytotoxicity in plant cell membranes. ADP and ATP active groups, sulphydryl (SH) group reactivity with cations, putative affinity for phosphate groups, and changes in cell membrane permeability may all be shocking. Repercussions High amounts of lead diminish floral production (Figure 7) [31].
Figure 7: Representation of the negative effects of lead on plants.
Lead’s mechanism of action
Symptoms similar to those of lead poisoning may also be produced by grass tetany, polioencephalomalcia (PEM), botulism, and plant poisoning (magnesium deficiency) All exposed cattle must have their lead residues examined [32].
Two more sources of lead in ecosystems are mining and direct waste stream discharge into water bodies. Environmental lead exposure may cause developmental issues and decreased growth. Vertebrate organisms such as plants and animals go through neurological consequences when reproducing. Lead poisoning may be brought on by unintentional intake of contaminated soil or dust as well as direct lead inhalation [33]. With the exception of some root vegetables like carrots, turnips, radishes, and beets, which take up very little lead in their stems and leaves, scientists say they are safe to consume. Most of the time, plants do not take the lead into their tissues. Particles may collect on produce grown in soil polluted with lead or in areas where lead-laced air pollution has accumulated. Consuming unclean produce might get you sick with the virus [34]. When inhaled or swallowed, lead particles may be harmful to one’s health. Healthy skin prevents the body from absorbing lead. Plants often do not absorb lead into their tissues. In regions where lead-laden air pollution has accumulated or on lead-contaminated soil, vegetables may pick up lead particles. Eating contaminated food may leave you open to infection (Figure 8). Little children who play in the dirt and then put their hands in their mouths run the risk of dying from lead exposure [11].
Figure 8: Representation of Mechanism of Action of Lead in Plants.
Prevention from Lead
The two primary treatment approaches are chelation therapy and lead source elimination. Treatment for lead poisoning also tackles deficiencies in iron, calcium, and zinc, all of which promote lead absorption. Whole intestine irrigation, cathartics, endoscopy, or even surgery may be utilized to remove any lead-containing particles found in the digestive tract and stop additional exposure [35]. Lead shrapnel and bullets might be harmful. If they’re close to fluid-filled or synovial regions, they may need to be surgically removed to avoid exposure. Treatment for organic lead poisoning involves removing the skin’s lead component, avoiding exposure, managing seizures, and maybe undergoing chelation therapy for elevated blood lead levels [36].
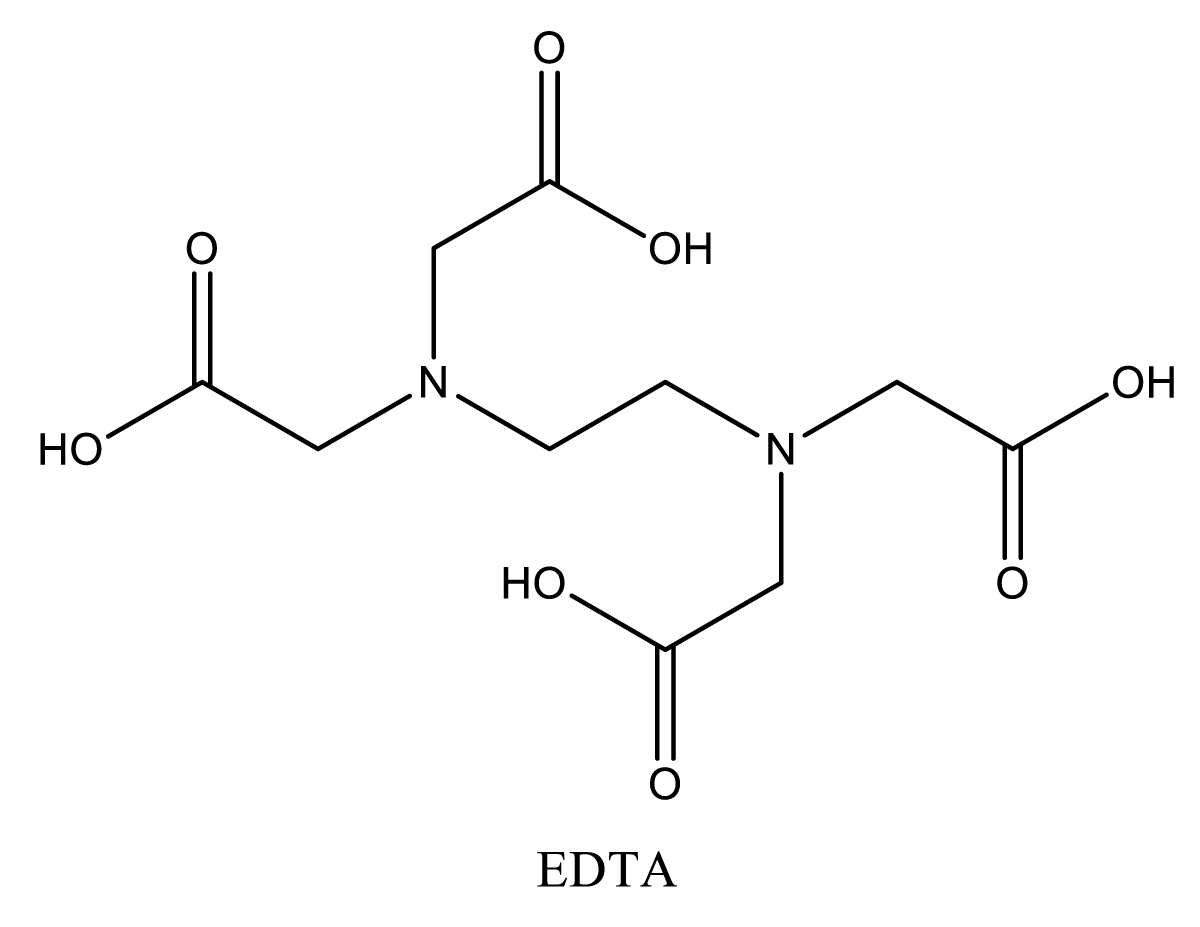
Since it has at least two groups that are negatively charged, lead, which is a chelating agent, may form complexes with metal ions that have multiple positive charges [37]. Urine may first eliminate the nontoxic chelate 50 times faster. Edetate disodium calcium (CaNa2EDTA), dimercaprol (BAL), succimer, and d-penicillamine are injectable and oral lead-poisoning chelating agents [37,38]. Chelation therapy is required for patients with acute lead poisoning, severe lead poisoning, or encephalopathy who have blood lead levels that are more than 25 g/dL [39]. Chelation therapy is not often administered to asymptomatic people who have high lead levels in their blood. Chelation therapy is unsuccessful for patients who have been exposed to persistently low levels of lead. If the patient’s symptoms improve or their blood lead levels return to normal, the chelation therapy is often discontinued. There is a possibility of an increase in blood lead levels after chelation due to lead leaking from bone reserves [40].
Mercury
The chemical element mercury has the atomic number 80 and the letter Hg [41]. Mercury is a heavy, silvery d-block element. It is the only metallic element that is liquid at these conditions. Cinnabar, the most prevalent kind of mercury, is present in deposits all over the planet (mercuric sulfide) [42].
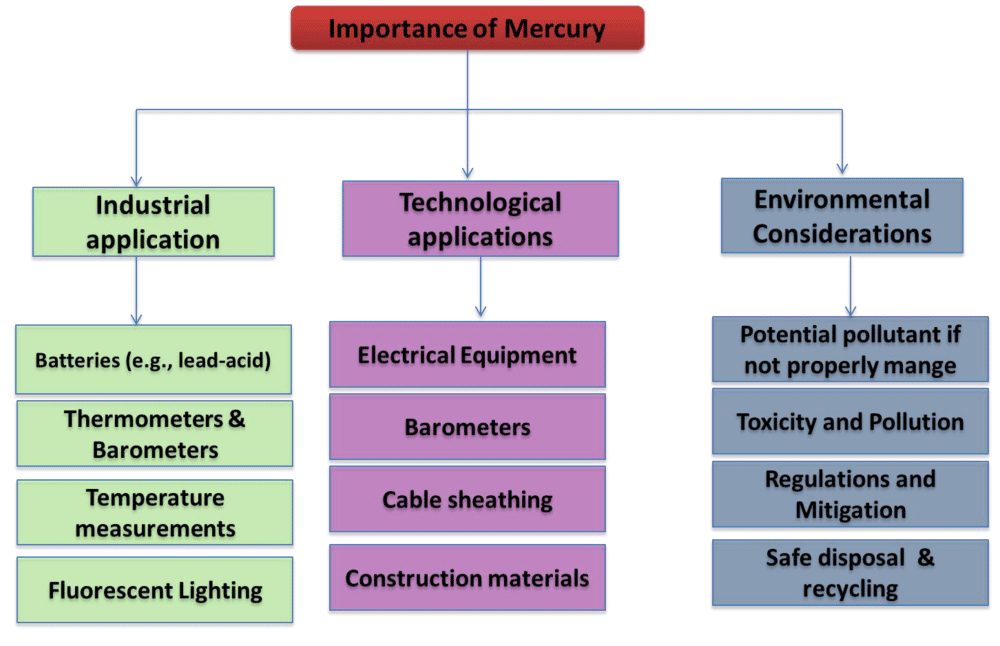
Grinding natural or synthetic mercuric produces vermilion. Thermometers, barometers, manometers, sphygmomanometers, float valves, mercury switches, mercury relays, fluorescent lights, and others use mercury. Mercury thermometers and sphygmomanometers have been phased out in clinical settings owing to concerns about their toxicity. Alcohol- or galinstan-filled glass thermometers and thermistor-based mechanical devices have replaced them [43]. The strain gauge sensors and digital pressure gauges have taken the position of mercury sphygmomanometers. In certain regions, dental amalgam and scientific research still employ mercury [44]. Moreover, fluorescent lighting uses it. A fluorescent lamp’s phosphor glows and emits visible light when electricity passes through the mercury vapour within the lamp, which then emits short-wave ultraviolet radiation [45] (Figure 9).
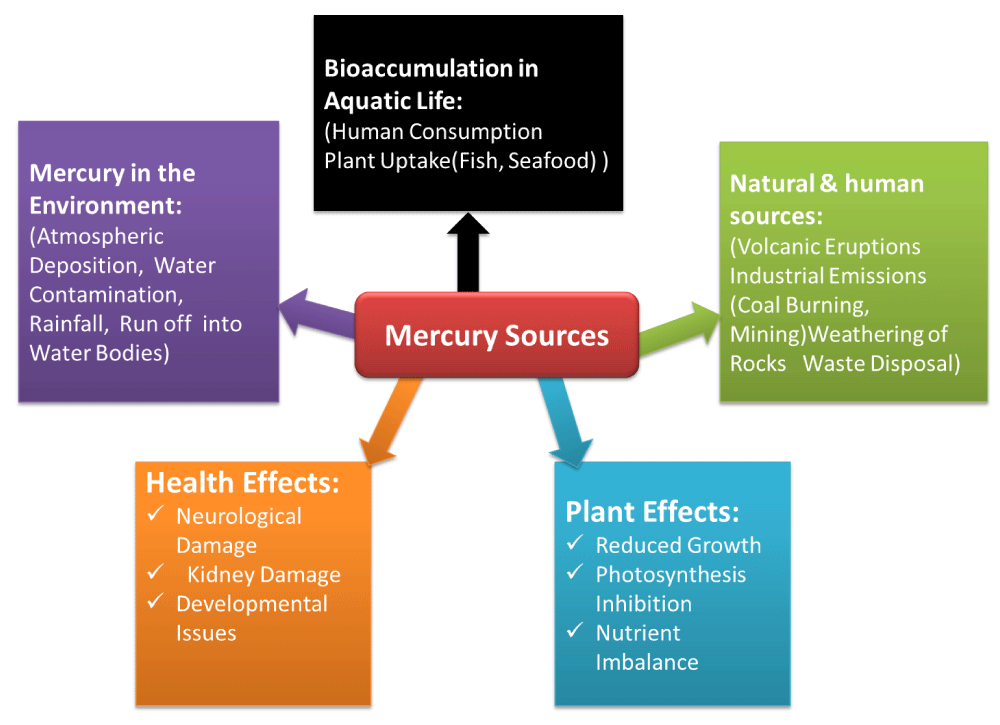
Figure 9: Representation of the sources, environmental pathways, and effects of mercury on both humans and plants.
Sources of mercury
Mercury is unique and released into the environment by natural and human activities. Mercury is the only liquid metal at room temperature. Nevertheless, it is generally solid or gas. Mercury, like other elements, can only change chemical states, making it very durable in the environment. We mostly worry about methylmercury since its chemical form determines its toxicity. Methylmercury in food chains harms humans and animals. Mercury enters the air, water, and soil via natural and human activities [46]. Granite and cinnabar contain mercury. Volcanic activity, undersea vents, and rock weathering release mercury from mineral deposits. Each year, natural sources release 2000 tonnes of mercury. Humans emit 2900 tonnes of mercury every year. Mercury emissions come from electricity production, transportation, and industry. mining, smelting, and burning [47]. These three sectors emit 70% of Canada’s mercury. Industrialization tripled mercury levels. Environmental mercury Mercury may travel across the planet once released into the atmosphere. Mercury is found in rain, snow, and dust, as well as around power plants and volcanoes. Elemental mercury dominates airborne mercury. Around 98% of atmospheric mercury is elemental. When deposited in the environment, elemental mercury may become methylmercury, which is more toxic [48]. Fish are often referenced in mercury’s environmental and health impacts. Fish are the main source of mercury for humans, and fish-eating animals like loons may accumulate enough mercury to damage their reproduction [49]. Large predatory fish have greater mercury levels than little fish that eat insects or plants. The purple bar’s size and darkness indicate the critters’ methylmercury levels. The large right fish has the most methylmercury [50].
Importance of mercury
Mercury is the only element and metal that can exist in a liquid state at normal room temperature and pressure (Hg). The raw form of mercury is extracted from the cinnabar ores, a granular, reddish-coloured deposit that is widespread across the globe [51]. Because of its solubility at room temperature and silvery appearance, it is sometimes referred to as “quicksilver.” In reality, certain small organisms transform elemental mercury into organic form when it is deposited in water (methylmercury). Eating fish and shellfish that contain methylmercury is the main way that mercury is ingested [52].
Methylmercury is particularly toxic to children and unborn people because it may harm their developing brains. Methylmercury exposure during pregnancy has been linked to impaired cognitive functioning, memory, attention, language, fine motor abilities, and visual-spatial skills in children [53,54]. Elemental breathing humans are also at risk for mercury-related illnesses. This kind of exposure may happen when items containing elemental mercury, such thermometers, malfunction inside with inadequate ventilation (Figure 10). More information about mercury poisoning and its treatment is available from the Centre for Disease Control and Prevention [55].
Figure 10: Representation of the importance and uses of mercury.
Harmful effects of mercury on plants
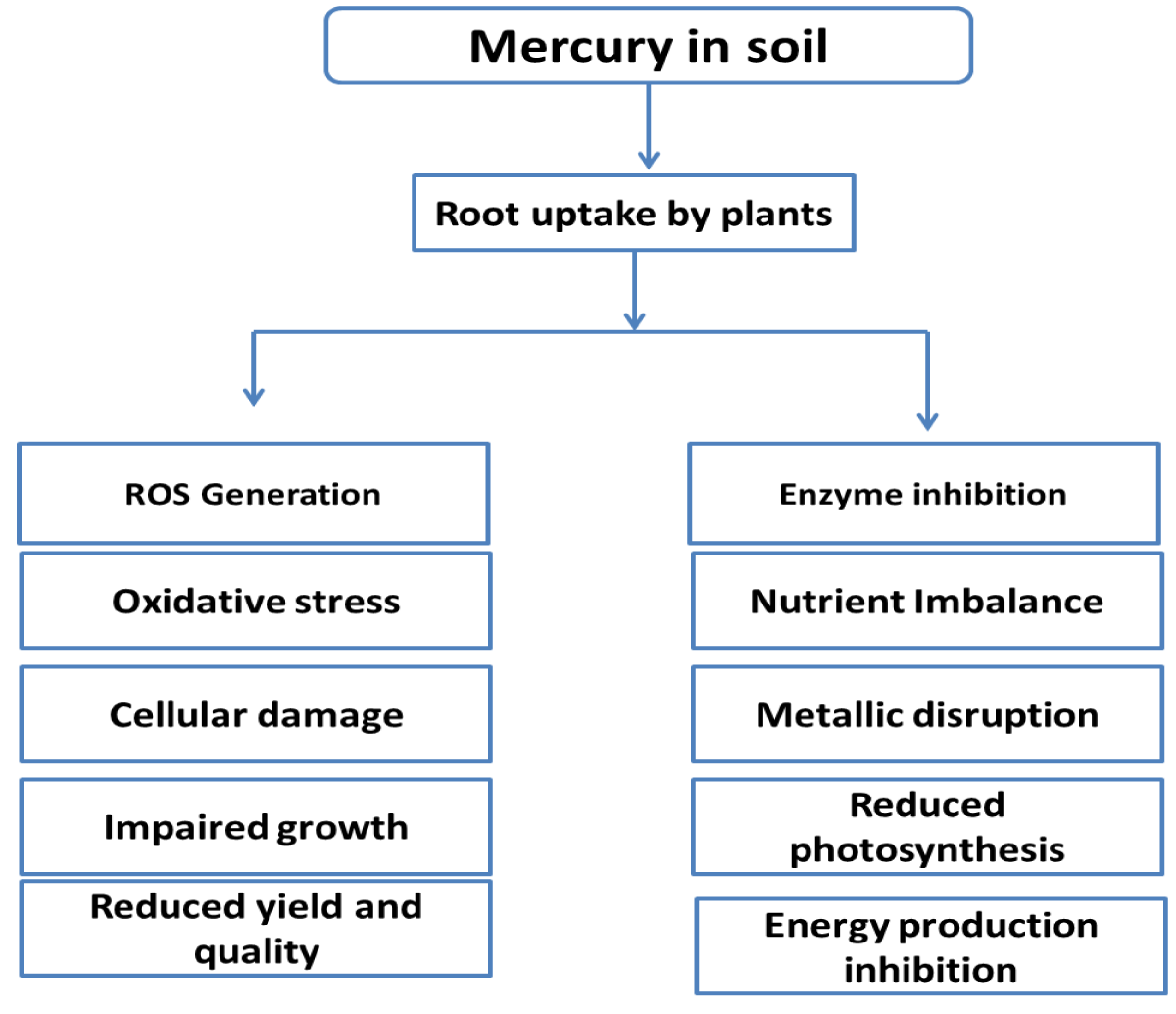
Heavy metals are a major environmental contaminant when they are found in soil at high levels and may impair plant growth, nodulation, nitrogen fixation, and other processes. When ingested, breathed, or absorbed via the skin, certain mercury compounds may result in neurological and behavioural issues. Just a few of the indications and symptoms include tremors, lack of sleep, memory loss, neuromuscular effects, headaches, and cognitive and motor dysfunction. Cellular structure is disturbed by mercury stress, and metabolism is still a mystery [56].
Both light and dark photosynthetic responses are impacted by mercury. In living cells, replacing the magnesium-containing core atom of chlorophyll with mercury prevents the absorption of photosynthetic light, which causes the breakdown of photosynthesis. Depending on how strong the light is, the impact varies [57].
Heavy metals are a major environmental contaminant when they are found in soil at high levels and may impair plant growth, nodulation, nitrogen fixation, and other processes [58]. Cellular structure is disturbed by mercury stress, and metabolism is still a mystery. Total soil mercury concentrations in the field had little effect on tree diversification. In the research we conducted in a greenhouse, it was discovered that organic mercury species are more dangerous than inorganic mercury species, and EFPC soil inoculants did not provide any protective effects against Hg toxicity [59,60] (Figure 11).
Figure 11: Representation of the Harmful Effects of Mercury on Plants.
Mercury’s mechanism of action
As it may damage the brain and reproductive systems of humans and other animals, mercury pollution of freshwater lakes, fish, and fish-eating birds like loons and eagles has long been known to occur [61]. Mercury has an impact on photosynthesis’s reactions to both light and darkness. In living organisms, replacing the magnesium-containing core atom of chlorophyll with mercury impairs the ability of those chlorophyll molecules to capture photosynthetic light, leading to the failure of photosynthesis. Depending on the brightness of the light, the response varies [62].
The most obvious and constant effect of spindle activity disruption is the formation of c-mitosis, which leads to polyploid and aneuploid cells as well as c-tumors. According to study, organomercurials are 200 times more potent than inorganic mercury. Exposure to inorganic mercury decreases the incidence of chromosomal aberrations and lowers the mitotic index in root-tip cells in a dose-dependent way. The relationship between exposure concentration and duration and the time it takes the body to heal after exposure to mercury is inverse. Toxic metal ion resistance systems, such as Hg2+, are encoded by bacterial plasmids and function by energy-dependent toxic metal ion efflux [63,64].
ATPases and chemiosmotic cation-proton antiporters are used to move ions. The inducible mercury resistance locus codes for both the detoxifying enzymes and the absorption of mercury ions. The periplasmic protein MerP, the inner-membrane transport protein MerT, the cytoplasmic enzyme mercuric reductase, the protein MerA, and the reduction of mercuric ions to elemental mercury, Hg, are all involved in the process in gram-negative bacteria (II). A mercury-sensitive acidophilic chemoautotrophic bacterium is called Thyobacillus ferrooxidans [65,66].
It has been discovered that a group of mercury-resistant strains known as ions may volatilize mercury. A 2.3 kb section of the chromosomal DNA from Escherichia coli strain E-l 5 that encodes 56,000 and 16,000 molecular-weight proteins, respectively, also contains the whole coding sequence for the mercury-ion resistance gene. In response to mercury heavy metal stress, higher plants and Schizosaccharomyces pombe produce phytochelatins (PCs) as chelators [67-69].
Mercury-safety
Everyone must contribute to mercury reduction. Purchase mercury-free thermostats, switches, relays, barometers, and manometers. Contact your local home hazardous trash collection programmed to recycle mercury-containing products and bulk mercury [70].
Mercury powers fluorescent and high-intensity-discharge lights. These lights are energy-efficient and use less electricity from coal-burning power stations, which release mercury into the atmosphere. Use and disposal of these commodities may limit mercury leakage from human activity [71].
Mercury-containing product manufacturers, government programmes, and solid waste disposal facilities have decreased mercury in the environment. Hennepin County’s waste-to-energy facility’s pollution control technology and procedures to keep mercury out of the waste stream lowered mercury emissions from 496 pounds in 1990 to 21 pounds in 2000, a 95% reduction. Mercury-free equipment include barometers, manometers, thermostats, switches, and relays [72,73].
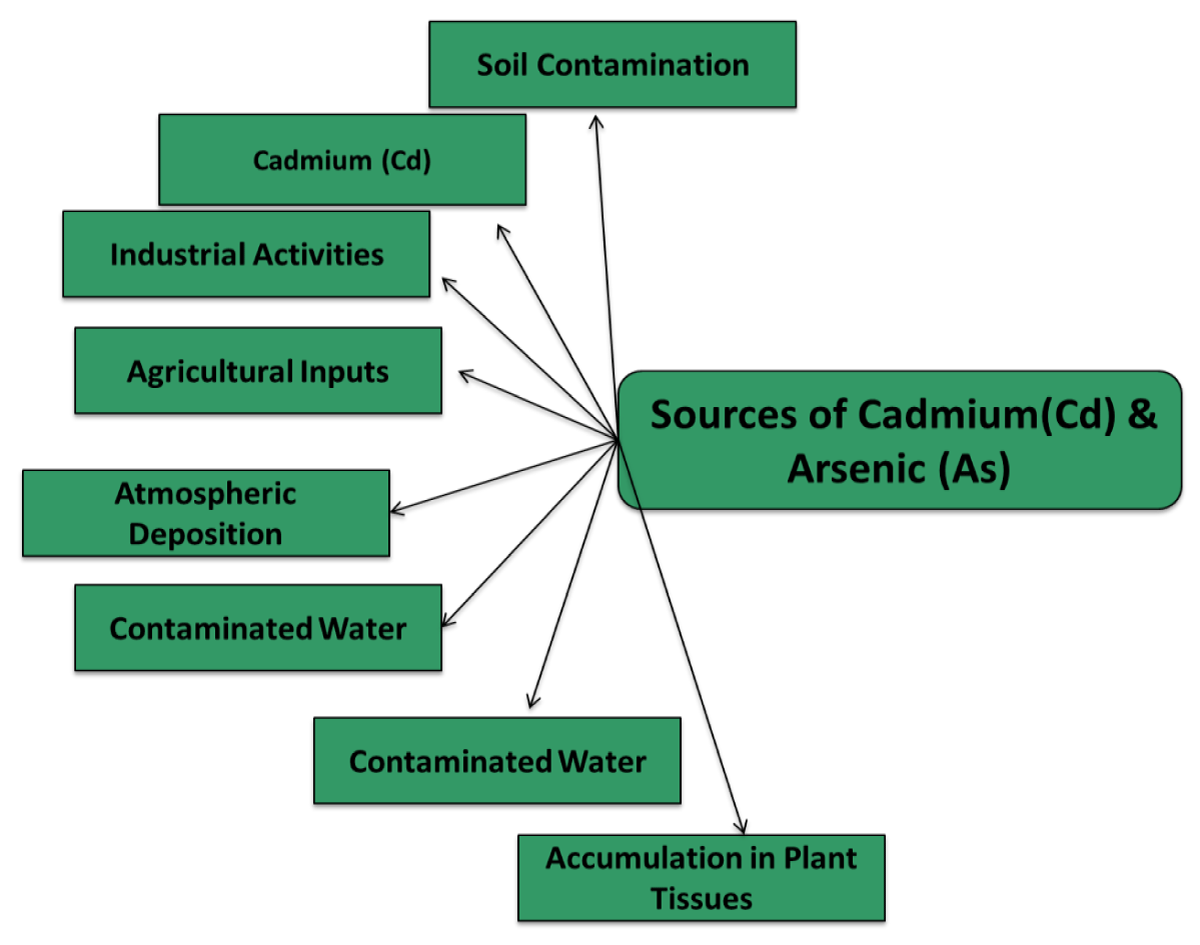
Cadmium and arsenic
Cadmium and arsenic sources in plants: Heavy metals have atomic numbers below 20 Cr, Hg, Cu, Cd, Pb, and As. Non-biodegradable materials impair biological systems. They harm soil microorganisms. Cadmium and arsenic are heavy metals [74]. Arsenic is a toxic metal with atomic weight 74.9, melting point 8170 C, and specific gravity 5.73. Several insecticides utilise it. - As has oxidation numbers 3, 0, +3, and +5. Industrial sediments and natural water sources contain it. Arsenic may occur as H3AsO3, H3AsO4, arsenites, methylarsenic acid, and arsenates. Natural waters include arsenite (AsO33-) and arsenate (AsO43-) [75-77].
It forms hard acid with oxides and nitrogen in soil. Trivalent arsenites develop in decreasing anaerobic conditions like ground water. As(OH)3, AsO2OH2-, As(OH)4-, and AsO33- are typical trivalent arsenates. For decades, researchers have studied As and Cd in paddy rice and tea crops. As and Cd cycling depends on PH, which is altered by electrons and protons. Due to paddy field flooding, As and Cd biogeochemical processes are impacted. As and Cd’s solubility is affected. Reduction potential decreases when rice fields flood due to microbial O2 consumption. When reduced, As and Cd on iron (oxy) hydroxide and manganese oxides release [75].
When As (v) is microbially reduced to As (III), its solubility decreases. When sulphate-reducing bacteria reduce sulphate to sulphide, cadmium sulphide may precipitate. CdS production decreases Cd solubility, although reduction of iron (oxy) hydroxides and As(v) to As enhances As solubility (III) [78].
After paddy water is drained, the process reverses (Cd solubility is increased). Cadmium creates soil-soluble complexes as a cationic element [79]. The major As (III) uptake route is silicon. Cadmium transporter OsNRAMP5 is manganese. It stands for natural resistance associated macrophage protein. Rice roots and exodermis and endodermis cells’ plasma membranes are polarized [80] (Figure 12).
Figure 12: Representation of the Sources of Cadmium and Arsenic in Plants.
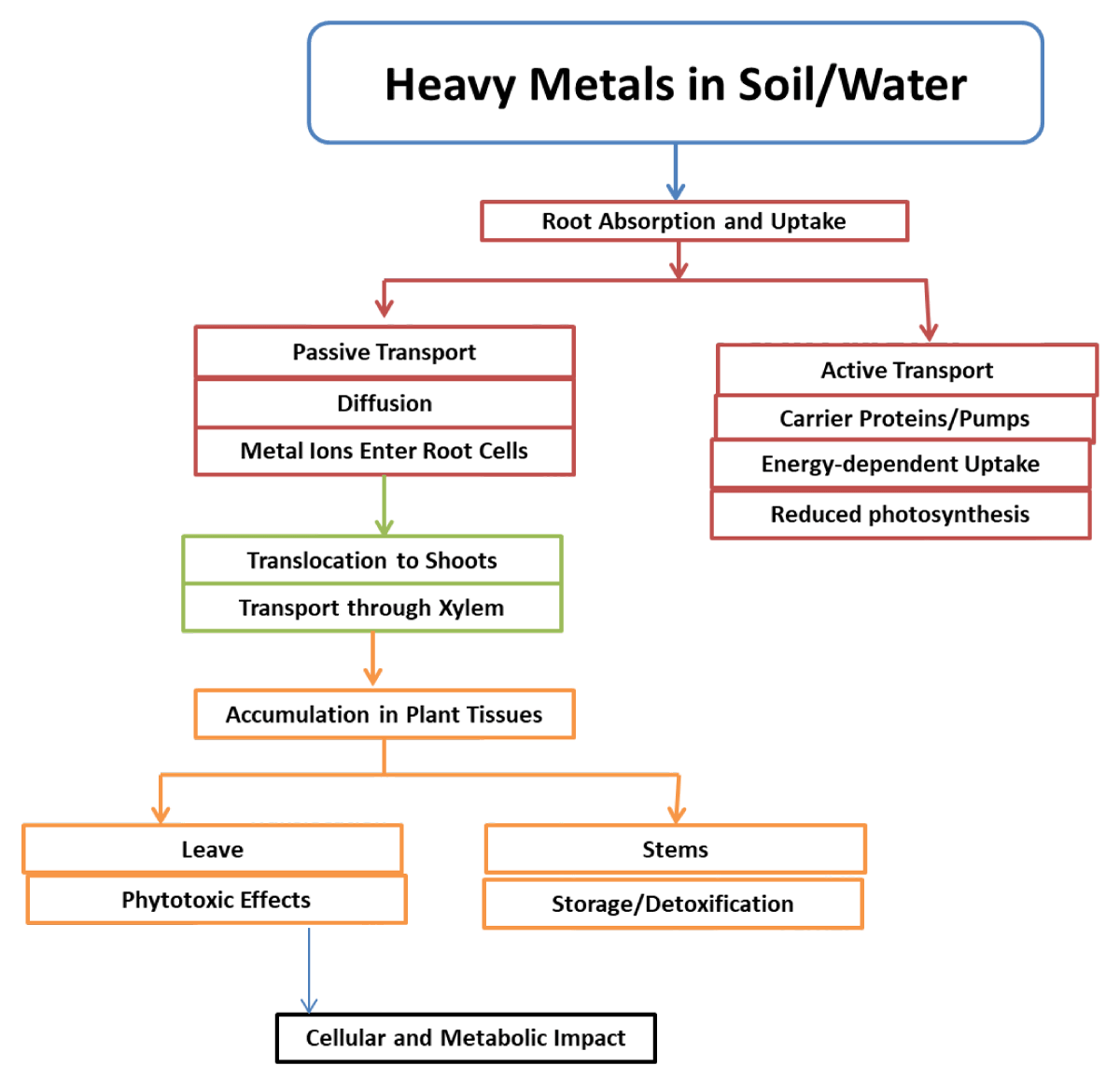
Mechanism of heavy metal uptake by plants
In order to get vital nutrients, plants use unique systems. Several of them, such redox potential, pH change, and chelating compounds produced by plants, have already been covered [81]. One example of a transport mechanism is a protein pump (also known as an ATPase, which generates an electrochemical gradient and uses energy), another is a co- and anti-transporter, which uses the electrochemical gradient created by ATPases, and a third is a channel that helps ions enter cells [82].
Most plants acquire trace elements in the range of 10 to 15 ppm depending on their needs, however there are certain plants that are hyperaccumulators that can store metals up to thousands of ppm. Evapotranspiration is the mechanism by which water molecules in roots are pulled by water transpiring from leaves. Following are several methods by which these heavy metals are absorbed [83] (Figure 13).
Figure 13: Representation of the key steps involved in the uptake and translocation of heavy metals by plants, highlighting both passive and active transport mechanisms.
Phyto extraction
It is the process by which plants move pollutants from the roots to the more exposed portions of the plant, such as the shoots and leaves [84].
Phyto stabilization
Absorption, accumulation of toxins in plant tissue, and precipitation within the root zone are all examples of this process, which is responsible for the immobilization of contaminants found in soil and groundwater [85].
Rhizo filtration
Since precipitation or adsorption of dissolved substances onto plant roots or adsorption into the roots may take place, this phenomenon is sometimes referred to as Rhizo-root [86].
Phytovolatilization
It is the process through which pollutants are absorbed by plants and subsequently flowed from the plants into the atmosphere. In this process, evapotranspiration is crucial [87].
Effects of cadmium and arsenic on plants
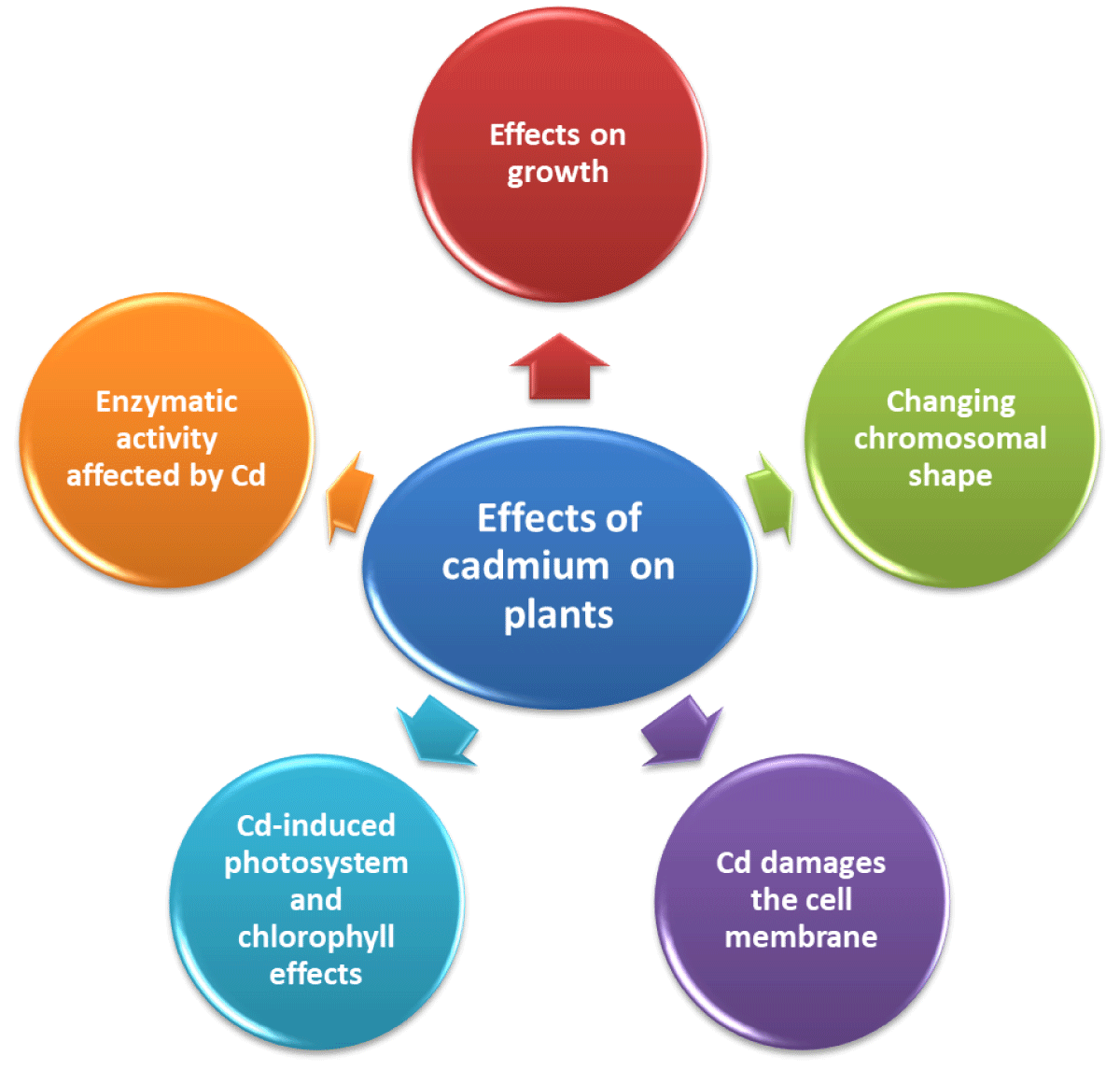
Effects of cadmium:• Effects on growth
Cadmium and other heavy metals are non-essential elements. Since cadmium accumulation negatively impacts metabolism, absorption, transport, and other components, it also has an impact on plant development [88].
The impact increases with concentration. Germination and growth rate in barley have both dropped by 45%. The primary targets of this metal are the roots, which causes harm to their development and slows them. Plants experience distinct affects at various phases of their lives. During the early stages of the rice plant, photosynthesis and the development of the reproductive organs were both inhibited. Chlorosis develops as a consequence of arsenic exposure’s suppression of inorganic nutrients [89,90].
• Changing chromosomal shape
The creation of this heavy metal causes errors in DNA synthesis, duplication, and chromosomal aberration. Zhang noticed in 1997 that Cd had an adverse impact on barley, causing nucleolus destruction and chromosomal aberration. There have been reports of nuclear decay, polyploidy, and chromosomal rings as adverse outcomes of exposure to Cd, Hg, and Pb [91].
• Cd damages the cell membrane
The enzymatic system serves as a barrier between the cell and its environment for the exchange of materials and information. Stabilization of enzymatic systems is required for the physiological operation of the cell. The damage caused by Cd to this enzyme system reduces the cell penetration. The polypeptide content of the thylakoid membrane is really modified under the stress of CD. The buildup of H2O2, O2, and malondialdehyde in wheat leaves reduced in the SH group, and electrolytic leakage of leaf cells was seen as the peroxidation of cellular membranes was sped up by active oxygen radicals [92].
• Cd-induced photosystem and chlorophyll effects
Chlorophyll proteins that absorb protons for photosynthesis in PS II are broken down by the Cd stress. As a result of the chloroplast’s sub-membrane structure changing, the membrane system is damaged. Thus, the drop-in protons have an impact on photosynthetic activity [93].
• Enzymatic activity affected by Cd
Superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) are protective enzymatic systems that are particularly effective in protecting against environmental stress. The balance of these three enzymes regulates the generation and destruction of free radicals, as well as the likelihood that free radicals can harm cells. The activity of these enzymes is reduced at high concentrations of heavy metals like Cd. Cellular damage is so increased [94] (Figure 14).
Figure 14: Representation of the effects of Cd on plants.
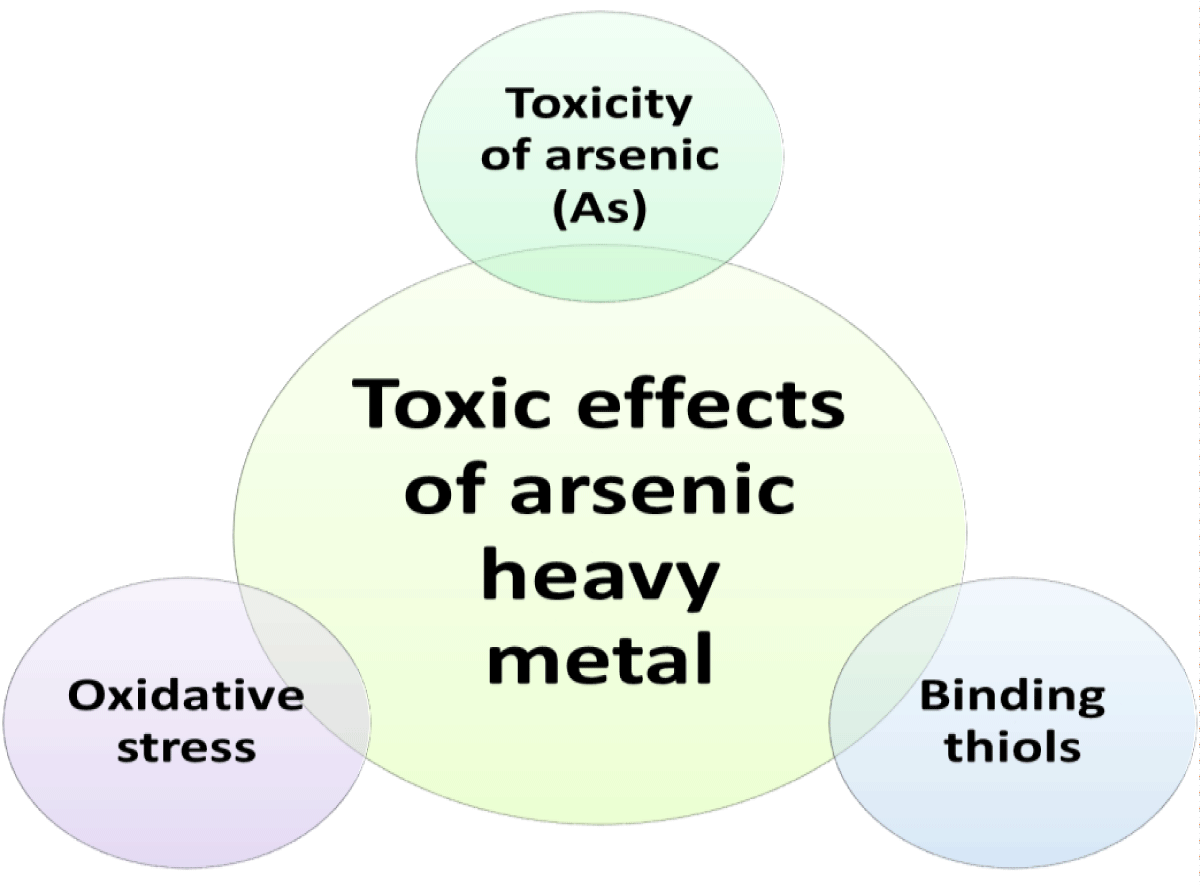
Toxic effects of arsenic heavy metal
Toxicity of arsenic (As): The mobility of various arsenic species varies, and as a result, so do their consequences. It has been discovered that the phytotoxic impact of As differs according on the kind of plant and the availability of nutrients [95]. Due to their structural similarity, arsenic may be used as a replacement for Pi in biochemical reactions, which disturbs several cellular functions. Similar to that, As III’s affinity for thiol groups in proteins and cofactors of enzymes makes it more likely to attach to them, putting cellular activities at risk. Reactive Oxygen Species (ROS) generation has been shown to be enhanced by arsenic [96].
Binding thiols: As III, which is a thiol reactive molecule that may bind up to three sulfohydral groups, acts as a cross linkage binding agent in contrast to As V. As III also binds poly-thiol compounds like PC in a similar manner [96]. Proteins and co-factors containing thiols are also bound by As III. AsIII targets the gly decarboxylase complex, 2-Oxoglutrate dehydrogenase complex, co factor dihydrolipoamide, and the mitochondrial and plastid pyruvate dehydrogenase complex. The half-life of a complex is just 1-2s, but as the number of bonds increases, so does stability, and half-lives may range from 1.3 min to 155 min when two or three thiol links are formed. Signal proteins, metabolic enzymes, and structural proteins are all directly impacted by the interaction of arsenics with thiols in proteins, which alters their folding and subsequently their structure [96,97].
Oxidative stress: Due to exposure to As III and V the formation of reactive oxygen species (ROS) such superoxide (O2-), H2O2, and hydroxy radicals (OH) increases. Membrane lipids may be peroxidized by ROS, and they can also harm proteins and purine nucleotides. Radicals produced from lipids are created as a result of lipid peroxidation. While the precise mechanism of ROS formation caused by As is unknown, PC synthesis and AsV to AsIII reductase are thought to be the primary causes [98].
Antioxidants already present in the body normally balance out ROS imbalance, but when exposed to As, ROS production increases, the body’s defenses are overpowered, and cellular damage results [99] (Figure 15).
Figure 15: Representation of the effects of Arsenics on plants.
Positive effects of Cd and As on plants
Osmolyte accumulation: While the buildup of As and Cd has certain harmful consequences, it also has some favourable ones. For instance, under As and Cd stress, maiz’s total protein and prolin levels were shown to rise by up to 30%. Similarly, a 34% rise in total soluble sugars has been seen in the maize variety Dong dang 80. Phenolic concentration rises by 40% and 49%, respectively, in the rung nong 35 and dong dang 80 varieties of maiz. The content of proteins, carbohydrates, and prolin increases throughout time [100,101].
Enzymatic and non-enzymatic antioxidants
Antioxidants that are both enzymatic and non-enzymatic are crucial in preventing the peroxidation of proteins and lipids brought on by ROS. Heavy metals like As and Cd do enhance these oxidants, but it has also been shown that they have an impact on enzymatic and non-enzymatic antioxidants. With the Dong dang 80 and Run nong 35 types of maiz, this effect has been researched. Both revealed a 20%-28% increase in all antioxidant species. Actually, As and Cd have an indirect relationship with the formation of these antioxidants since the metals create a rise in ROS, which is directly tied to the creation of antioxidants [101,102].
Preventions from heavy metals As and Cd
Induced proteins and peptides: Metal-binding proteins and organic acid chelation lessen heavy metal stress. He and Luo isolated maiz Cd-binding protein in 1991 at a 1:3 ratio. Metal binding proteins, a natural plant resistance to heavy metals like As and Cd, bind 40% - 45% of Cd, reducing its harm to 40% - 45%. Rice, beans, and tobacco now yield metal-binding proteins such metallothiopeptides. They may be planted. Phytochelatin induces another heavy metal detoxifier. PC is produced by phyto chelatin synthase catalyzing glutathione, not gene translation [103].
Enzyme and nucleic acid gene expression
Another enzyme found in plants that combines with heavy metals is lacti-dehyrogenase. The alcoholic dehydrogenase (ADH) alteration has been noticed on two levels as well as Cd. When various heavy metals are introduced to plants, DNA-like proteins are created that can be removed from the plant and added to other heavy metal-stressed plants [104].
The role of Heavy Metals (HMs) in plant life is a complex and multifaceted subject that has garnered significant attention in recent years. However, gaps remain in the existing literature, particularly in the integration of both the beneficial and detrimental effects of HMs on plants. Much of the research to date has tended to focus either on the essential roles of HMs in plant nutrition or on their toxicity and the associated stress responses, often treating these aspects separately. This fragmented approach fails to fully capture the dynamic interactions between HMs and plant systems across diverse environmental contexts. This review addresses these gaps by synthesizing current knowledge on the dual roles of HMs, providing a balanced perspective that considers both their essentiality and toxicity. By doing so, it offers a more comprehensive understanding of how plants interact with HMs, highlighting the need for strategies that can harness the benefits of HMs while mitigating their risks.
From the author’s perspective, recognizing the dual nature of HMs is crucial for developing sustainable agricultural practices that can adapt to the increasing levels of HMs in the environment. This review emphasizes the importance of adopting an integrative approach that considers both the beneficial and harmful impacts of HMs, which is essential for informed decision-making in environmental management and agriculture. However, alternative perspectives from various scholars suggest a more focused approach, either by enhancing the beneficial roles of HMs through biofortification or by prioritizing efforts to mitigate HM toxicity. While these approaches have merit, they may not fully address the broader ecological implications and the need for a nuanced understanding of HMs’ interactions with plant systems.
The limitations of this review include its reliance on studies published predominantly in English, which may overlook significant research conducted in other languages, and the focus on literature from 2000 to 2023, potentially missing out on earlier foundational studies. Additionally, while the review provides a comprehensive overview, it does not delve into the specific molecular mechanisms of HM tolerance and toxicity, which could be an area for further exploration. Future research should aim to bridge these gaps by exploring the effects of HMs across a wider range of plant species and ecosystems, particularly in under-researched regions and environments. Moreover, interdisciplinary studies that integrate molecular biology, environmental science, and agronomy are needed to develop more effective and sustainable strategies for managing HM contamination in agricultural systems. By addressing these areas, future research can contribute to a deeper understanding of HMs’ roles in plant life and support the development of innovative solutions to the challenges posed by HM pollution.
We may draw the following conclusion from the above conversation: heavy metals have benefits as well as drawbacks. We are unable to dispute the usefulness and significance of these things in both everyday life and the functioning of living systems. But, they also pose a danger to the lives of both plants and animals (both aquatic and terrestrial animals). They are the cause of numerous illnesses that affect both plants and animals. That indicates that we need to make use of these metals, but in addition to that, we ought to take preventative precautions. If we don’t take the necessary safeguards, then heavy metals will contaminate our environment, and the range of consequences they have will be far wider. We need to devise such processes so that we may utilize these metals while causing a minimum amount of harm to the surrounding ecosystem.
Although AI-generated tools were used to generate this eBook/ Article, the concepts and central ideas it contains were entirely original and devised by a human writer. The AI merely assisted in the writing process, but the creative vision and intellectual property belong to the human author.
- Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. In: Molecular, Clinical and Environmental Toxicology: Volume 3: Environmental Toxicology. 2012:133-64. Available from: https://doi.org/10.1007/978-3-7643-8340-4_6
- Lang PF. Bonding, structure and uses of metals. J Metal Mater Res. 2023;6(1). Available from: https://doi.org/10.30564/jmmr.v6i1.5173
- Wang B, Lan J, Bo C, Gong B, Ou J. Adsorption of heavy metal onto biomass-derived activated carbon. RSC Adv. 2023;13(7):4275-302. Available from: https://doi.org/10.1039%2Fd2ra07911a
- Kumari S, Mishra A. Heavy metal contamination. In: Soil Contamination-Threats and Sustainable Solutions. IntechOpen; 2021. Available from: https://www.intechopen.com/chapters/72968
- Madhu PM, Sadagopan RS. Effect of heavy metals on growth and development of cultivated plants with reference to cadmium, chromium and lead–a review. J Stress Physiol Biochem. 2020;16(3):84-102. Available from: https://web.archive.org/web/20201124230326id_/http://www.jspb.ru/issues/2020/N3/JSPB_2020_3_84-102.pdf
- Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M. Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol. 2021;12:227. Available from: https://doi.org/10.3389%2Ffphar.2021.643972
- Vetrimurugan E, Brindha K, Elango L, Ndwandwe OM. Human exposure risk to heavy metals through groundwater used for drinking in an intensively irrigated river delta. Appl Water Sci. 2017;7:3267-3280. Available from: https://ui.adsabs.harvard.edu/link_gateway/2017ApWS....7.3267V/doi:10.1007/s13201-016-0472-6
- Qin G, Niu Z, Yu J, Li Z, Ma J, Xiang P. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere. 2021;267:129205. Available from: https://doi.org/10.1016/j.chemosphere.2020.129205
- Miller R. The elements: what you really want to know. Twenty-First Century Books; 2006. Available from: https://librarycatalog.cityofwoodland.gov/Record/.b17940795
- Boldyrev M. Lead: properties, history, and applications. WikiJ Sci. 2018;1(2):1-23. Available from: https://doi.org/10.15347/wjs%2F2018.007
- Ara A, Usmani JA. Lead toxicity: a review. Interdiscip Toxicol. 2015;8(2):55-64. Available from: https://doi.org/10.1515%2Fintox-2015-0009
- Levin R, Vieira CLZ, Rosenbaum MH, Bischoff K, Mordarski DC, Brown MJ. The urban lead (Pb) burden in humans, animals and the natural environment. Environ Res. 2021;193:110377. Available from: https://doi.org/10.1016/j.envres.2020.110377
- Jones FN, Nichols ME, Pappas SP. Organic coatings: science and technology. John Wiley & Sons. 2017. Available from: https://onlinelibrary.wiley.com/doi/book/10.1002/9781119337201
- Dignam T, Kaufmann RB, LeStourgeon L, Brown MJ. Control of lead sources in the United States, 1970-2017: public health progress and current challenges to eliminating lead exposure. J Public Health Manag Pract. 2019;25(Suppl 1 LEAD POISONING PREVENTION). Available from: https://doi.org/10.1097%2FPHH.0000000000000889
- Clausen J, Bostick B, Korte N. Migration of lead in surface water, pore water, and groundwater with a focus on firing ranges. Crit Rev Environ Sci Technol. 2011;41(15):1397-1448. Available from: http://dx.doi.org/10.1080/10643381003608292
- Siegmund A. Primary lead production–a survey of existing smelters. In: Lead-Zinc. 2000:53-116. Available from: https://gcteng.com/wp-content/uploads/2000_Primary_Lead_Survey.pdf
- WATER D, ENTERS HL. Lead in drinking water. 2012.
- Dunn DJ. Water Delivery Report 2008. 2008. Available from: https://curaflo.com/wp-content/uploads/2017/07/CuraFloWaterDeliveryrReport2008-3_0.pdf
- Maas RP, Patch SC, Morgan DM, Pandolfo TJ. Reducing lead exposure from drinking water: recent history and current status. Public Health Rep. 2005;120(3):316-321. Available from: https://doi.org/10.1177%2F003335490512000317
- Venkatesh DT, Paul A. Lead testing in soil contaminated with lead and reducing its effects on humans by the activity of activated lead. Available from: https://www.researchgate.net/publication/303387938_LEAD_TESTING_IN_SOIL_CONTAMINATED_WITH_LEAD_AND_REDUCING_ITS_EFFECTS_OF_HUMAN_BY_THE_ACTIVITY_OF_ACTIVATED_LEAD
- Suliman NOF. Factors affecting child health with focus on lead element pollution: a case study of Greater Wad Medani Locality Gezira State-Sudan (2017). University of Gezira; 2018. Available from:
- Singh R, Parthvi R, Parthvi P, Agarwal J, Gupta P. Heavy metal contamination of spices. 2013; 3(1):47-65. Available from: https://www.researchgate.net/publication/320211984_HEAVY_METAL_CONTAMINATION_OF_SPICES
- Slade G. Made to break: Technology and obsolescence in America. Harvard University Press; 2006. Available from: https://books.google.co.in/books/about/Made_to_Break.html?id=YMoxdac6J-cC&redir_esc=y
- Hunt WG, Crum B, Watson RT, et al. Lead bullet fragments in venison from rifle-killed deer: potential for human dietary exposure. PLoS One. 2009;4(4).
- Zhang H, F Wang, Y Lu, Q Sun, Y Xu, BB Zhang, et al. High-sensitivity X-ray detectors based on solution-grown caesium lead bromide single crystals. J Mater Chem C. 2020;8(4):1248-1256. Available from: https://pubs.rsc.org/en/content/articlelanding/2020/tc/c9tc05490a
- Juberg DR, Kleiman CF, Kwon SC. Position paper of the American Council on Science and Health: lead and human health. Ecotoxicol Environ Saf. 1997;38(3):162-180. Available from: https://doi.org/10.1006/eesa.1997.1591
- Hossain MA, Piyatida P, da Silva JAT, Fujita M. Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J Botany. 2012;2012:1-19. Available from: https://doi.org/10.1155/2012/872875
- Fahr M, Popp M, Schubert S. Effect of lead on root growth. Front Plant Sci. 2013;4:175. Available from: https://doi.org/10.3389/fpls.2013.00175
- Emamverdian A, Ding Y, Mokhberdoran F, Xie Y. Heavy metal stress and some mechanisms of plant defense response. Sci World J. 2015;2015:1-9. Available from: https://doi.org/10.1155%2F2015%2F756120
- Tuteja N, Gill SS, Tuteja R. Plant responses to abiotic stresses: shedding light on salt, drought, cold and heavy metal stress. In: Omics and Plant Abiotic Stress Tolerance. 2011;1:39-64. Available from: http://dx.doi.org/10.2174/97816080505811110101
- Aslam R, Bhat TM, Choudhary S, Ansari M. An overview on genotoxicity of heavy metal in a spice crop (Capsicum annuum L.) in respect to cyto-morphological behaviour. Caryologia. 2017;70(1):42-47. Available from: https://doi.org/10.1080/00087114.2016.1258884
- McAllister TA, Ribeiro G, Stanford K, Wang Y. Forage-induced animal disorders. In: Forages: The Science of Grassland Agriculture. 2020;2:839-860. Available from: https://www.scirp.org/reference/referencespapers?referenceid=3318471
- Pandey G, Madhuri S. Heavy metals causing toxicity in animals and fishes. Res J Anim Vet Fish Sci. 2014;2(2):17-23. Available from: https://www.researchgate.net/publication/303213699_Heavy_metals_causing_toxicity_in_animals_and_fishes
- Knez E, Kadac-Czapska K, Dmochowska-Ślęzak K, Grembecka M. Root vegetables—composition, health effects, and contaminants. Int J Environ Res Public Health. 2022;19(23):15531. Available from: https://doi.org/10.3390/ijerph192315531
- Gracia RC, Snodgrass WR. Lead toxicity and chelation therapy. Am J Health Syst Pharm. 2007;64(1):45-53. Available from: https://doi.org/10.2146/ajhp060175
- Mosheiff R, Weil Y, Khoury A, Liebergall M. The use of computerized navigation in the treatment of gunshot and shrapnel injury. Comput Aided Surg. 2004;9(1-2):39-43. Available from: https://doi.org/10.3109/10929080400006382
- Neubauer U, Furrer G, Kayser A, Schulin R. Siderophores, NTA, and citrate: potential soil amendments to enhance heavy metal mobility in phytoremediation. Int J Phytoremediation. 2000;2(4):353-68. Available from: http://dx.doi.org/10.1080/15226510008500044
- Hauptman M, Bruccoleri R, Woolf AD. An update on childhood lead poisoning. Clin Pediatr Emerg Med. 2017;18(3):181-192. Available from: https://doi.org/10.1016%2Fj.cpem.2017.07.010
- Committee on Environmental Hazards, Committee on Accident Prevention, Committee on Environmental Health. Statement on childhood lead poisoning. Pediatrics. 1987;79(3):457-465. Available from: https://pubmed.ncbi.nlm.nih.gov/3822655/
- Lowry JA. Oral chelation therapy for patients with lead poisoning. Am Acad Pediatr. 2010;116:1036-1046. Available from: https://jeffreydachmd.com/wp-content/uploads/2015/07/Lead_Oral_Chelators_J_A_Lowry_2010.pdf
- Lin C-J, Pehkonen SO. The chemistry of atmospheric mercury: a review. Atmos Environ. 1999;33(13):2067-2079. Available from: http://dx.doi.org/10.1016/S1352-2310(98)00387-2
- Sidhaarth DK, Baskar S. A review on adsorption of nickel and mercury from aqueous solution using nanoparticles. Int J Civil Eng Technol. 2018;9(9): 1246-1255. Available from: https://www.researchgate.net/publication/362668219_A_Review_on_Adsorption_of_Nickel_and_Mercury_from_Aqueous_Solution_using_Nanoparticles
- Trüeb RM, Trüeb RM. The hair cycle and its relation to nutrition. In: Nutrition for Healthy Hair: Guide to Understanding and Proper Practice. 2020:37-109. Available from: https://www.amazon.in/Nutrition-Healthy-Hair-Understanding-Practice/dp/3030599191
- Blue LY. Immobilization of mercury and arsenic through covalent thiolate bonding for the purpose of environmental remediation. University of Kentucky; 2010. Available from: https://uknowledge.uky.edu/cgi/viewcontent.cgi?params=/context/gradschool_diss/article/1788/&path_info=Blue_Diss_FINAL.pdf
- Khanna VK. Fundamentals of solid-state lighting: LEDs, OLEDs, and their applications in illumination and displays. CRC Press; 2014. Available from: https://doi.org/10.1201/b17076
- He F, Gao J, Pierce E, Strong P, Wang H, Liang L. In situ remediation technologies for mercury-contaminated soil. Environ Sci Pollut Res. 2015;22:8124-8147. Available from: https://doi.org/10.1007/s11356-015-4316-y
- Gomes CS, Silva EA. Health benefits and risks of minerals: bioavailability, bio-essentiality, toxicity, and pathologies. In: Minerals latu sensu and Human Health: Benefits, Toxicity and Pathologies. Springer; 2021; 81-179. Available from: http://dx.doi.org/10.1007/978-3-030-65706-2_4
- Kim M-K, Zoh K-D. Fate and transport of mercury in environmental media and human exposure. J Prev Med Public Health. 2012;45(6):335. Available from: https://doi.org/10.3961%2Fjpmph.2012.45.6.335
- Desforges JPW, Yurkowski DJ, Nilsen EA, et al. Mercury and neurochemical biomarkers in multiple brain regions of five Arctic marine mammals. Neurotoxicol. 2021;84:136-145. Available from: https://doi.org/10.1016/j.neuro.2021.03.006
- MacDonald T. Effects of inorganic mercury on developing zebrafish (Danio rerio) larvae. University of Saskatchewan; 2015. Available from: https://core.ac.uk/download/pdf/226135712.pdf
- Chauke T. Geology and geochemistry of Muyexe magnesite deposit, Giyani Greenstone Belt, Limpopo Province, South Africa. 2020. Available from: https://univendspace.univen.ac.za/handle/11602/1624
- Khan MA, Siddiqi ZA. Mercury: sources, toxicity and remediation. In: Heavy Metals.1.
- Antonelli MC, Pallarés ME, Ceccatelli S, Spulber S. Long-term consequences of prenatal stress and neurotoxicants exposure on neurodevelopment. Prog Neurobiol. 2017;155:21-35. Available from: https://doi.org/10.1016/j.pneurobio.2016.05.005
- Gil A, Gil F. Fish, a Mediterranean source of n-3 PUFA: benefits do not justify limiting consumption. Br J Nutr. 2015;113(S2). Available from: https://doi.org/10.1017/s0007114514003742
- Atti SK, Cushing T, Oladele A, Casteel S, Kiernan E, Dela Cruz R, et al. All that glitters is not gold: mercury poisoning in a family mimicking an infectious illness. Curr Probl Pediatr Adolesc Health Care. 2020;50(2):100758. Available from: https://doi.org/10.1016/j.cppeds.2020.100758
- Stambulska UY, Bayliak MM, Lushchak VI. Chromium (VI) toxicity in legume plants: modulation effects of rhizobial symbiosis. Biomed Res Int. 2018;2018:1-8. Available from: https://doi.org/10.1155/2018/8031213
- Liu X. Chlorin-like photosensitizers for photodynamic therapy. University of British Columbia; 2005. Available from: https://open.library.ubc.ca/soa/cIRcle/collections/ubctheses/831/items/1.0061112
- Dary M, Chamber-Pérez MA, Palomares A, Pajuelo E. “In situ” phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria. J Hazard Mater. 2010;177(1-3):323-330. Available from: https://doi.org/10.1016/j.jhazmat.2009.12.035
- Mani D, Kumar C. Biotechnological advances in bioremediation of heavy metals contaminated ecosystems: an overview with special reference to phytoremediation. Int J Environ Sci Technol. 2014;11:843-872. Available from: http://dx.doi.org/10.1007/s13762-013-0299-8
- Jean-Philippe SR, Franklin JA, Buckley DS, Hughes K. The effect of mercury on trees and their mycorrhizal fungi. Environ Pollut. 2011;159(10):2733-2739. Available from: https://doi.org/10.1016/j.envpol.2011.05.017
- Chan H, Scheuhammer A, Ferran A, Loupelle C, Holloway J, Weech S. Impacts of mercury on freshwater fish-eating wildlife and humans. Hum Ecol Risk Assess. 2003;9(4):867-883. Available from: http://dx.doi.org/10.1080/713610013
- Desrosiers M, Planas D, Mucci A. Mercury methylation in the epilithon of boreal shield aquatic ecosystems. Environ Sci Technol. 2006;40(5):1540-1546. Available from: http://dx.doi.org/10.1021/es0508828
- Sharma S, Nagpal A, Vig AP. Genoprotective potential of Brassica juncea (L.) Czern. against mercury-induced genotoxicity in Allium cepa L. Turk J Biol. 2012;36(6):622-629. Available from: https://journals.tubitak.gov.tr/cgi/viewcontent.cgi?article=1740&context=biology
- Agarwal M, Rathore RS, Jagoe CH, Chauhan A. Multiple lines of evidences reveal mechanisms underpinning mercury resistance and volatilization by Stenotrophomonas sp. MA5 isolated from the Savannah River Site (SRS), USA. Cells. 2019;8(4):309. Available from: https://doi.org/10.3390/cells8040309
- Silver S, Phung LT. Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol. 1996;50:753-789. Available from: https://doi.org/10.1146/annurev.micro.50.1.753
- Barkay T, Miller SM, Summers AO. Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiol Rev. 2003;27(2-3):355-384. Available from: https://doi.org/10.1016/s0168-6445(03)00046-9
- Gatehouse LN. Construction of a cDNA library encoding pea seed proteins. Durham University; 1985. Available from: https://etheses.dur.ac.uk/7118/
- Gupta DK, Vandenhove H, Inouhe M. Role of phytochelatins in heavy metal stress and detoxification mechanisms in plants. In: Heavy metal stress in plants. 2013; 73-94. Available from: http://dx.doi.org/10.1007/978-3-642-38469-1_4
- Yadav SK. Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot. 2010;76(2):167-179. Available from: https://doi.org/10.1016/j.sajb.2009.10.007
- I I I H Works. A guide to mercury reduction in industrial and commercial settings. 2001. Available from: https://archive.epa.gov/region5/mercury/web/pdf/delta%20inst%20merc%20red%20guide%20ind%20comm%2001.pdf
- Lee K. 2022 Solid-state lighting R&D opportunities. Guidehouse; 2022. Available from: https://www.energy.gov/sites/default/files/2022-02/2022-ssl-rd-opportunities.pdf
- Cheng H, Hu Y. Mercury in municipal solid waste in China and its control: a review. Environ Sci Technol. 2012;46(2):593-605. Available from: https://doi.org/10.1021/es2026517
- Cabaniss AD. Handbook on household hazardous waste. Rowman & Littlefield; 2018. Available from: https://books.google.co.in/books?hl=en&lr=&id=745iDwAAQBAJ&oi=fnd&pg=PP2&dq=73.%09Cabaniss+AD.+Handbook+on+household+hazardous+waste.+Rowman+%26+Littlefield%3B+2018&ots=WJZsBTglJL&sig=NtH2bD50zQFmwDeKrr5xwxplQmk&redir_esc=y#v=onepage&q=73.%09Cabaniss%20AD.%20Handbook%20on%20household%20hazardous%20waste.%20Rowman%20%26%20Littlefield%3B%202018&f=false
- Shuaib M, Azam N, Bahadur S, Romman M, Yu Q, Xuexiu C. Variation and succession of microbial communities under the conditions of persistent heavy metal and their survival mechanism. Microb Pathog. 2021;150:104713. Available from: https://doi.org/10.1016/j.micpath.2020.104713
- Fatoki OS, Akinsoji OS, Ximba BJ, Olujimi O, Ayanda OS. Arsenic contamination: Africa the missing gap. 2013; 25: 16. Available from: https://doi.org/10.14233/ajchem.2013.15360
- Mou SA, Kabir MH, Yasmin S, Ahmed S. Arsenic mitigation technologies from ground water: a brief review.2022; 2(5):139-158. Available from: http://dx.doi.org/10.34104/ajpab.020.01390158
- Letsoalo MR. Speciation of arsenic water and sediments from Mokolo and Great Letaba Rivers, Limpopo Province. 2017. Available from: http://hdl.handle.net/10386/1927
- Du Laing G, Rinklebe J, Vandecasteele B, Meers E, Tack FM. Trace metal behaviour in estuarine and riverine floodplain soils and sediments: a review. Sci Total Environ. 2009;407(13):3972-3985. Available from: https://doi.org/10.1016/j.scitotenv.2008.07.025
- Kulsum PG, Khanam R, Das S, Nayak AK, Tack FMG, Meers E, et al. A state-of-the-art review on cadmium uptake, toxicity, and tolerance in rice: from physiological response to remediation process. Environ Res. 2022;115098. Available from: https://doi.org/10.1016/j.envres.2022.115098
- Ishimaru Y, Takahashi R, Bashir K, Shimo H, Senoura T, Sugimoto K, et al. Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport. Sci Rep. 2012;2(1):286. Available from: https://doi.org/10.1038%2Fsrep00286
- Frossard E, Bucher M, Mächler F, Mozafar A, Hurrell RF. Potential for increasing the content and bioavailability of Fe, Zn and Ca in plants for human nutrition. J Sci Food Agric. 2000;80(7):861-79. Available from: http://dx.doi.org/10.1002/(SICI)1097-0010(20000515)80:7%3C861::AID-JSFA601%3E3.3.CO;2-G
- Yang Y, Xie J, Li J, Zhang J, Zhang X, Yao Y, Wang C, et al. Trehalose alleviates salt tolerance by improving photosynthetic performance and maintaining mineral ion homeostasis in tomato plants. Front Plant Sci. 2022;13:974507. Available from: https://doi.org/10.3389/fpls.2022.974507
- Tangahu BV, Sheikh Abdullah SR, Basri H, Idris M, Anuar N, Mukhlisin M. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int J Chem Eng. 2011;2011:939161. Available from: http://dx.doi.org/10.1155/2011/939161
- Alamo-Nole L, Su YF. Translocation of cadmium in Ocimum basilicum at low concentration of CdSSe nanoparticles. Appl Mater Today. 2017;9:314-318. Available from: https://doi.org/10.1007%2Fs40201-022-00822-1
- Bolan NS, Park JH, Robinson B, Naidu R, Huh KY. Phytostabilization: a green approach to contaminant containment. Adv Agron. 2011;112:145-204. Available from: https://research-repository.uwa.edu.au/en/publications/phytostabilization-a-green-approach-to-contaminant-containment
- Thirkell TJ. The influence of nitrogen source on the nutrition of arbuscular mycorrhizal plants. University of York; 2016. Available from: https://etheses.whiterose.ac.uk/17272/1/Thomas%20James%20Thirkell%20Thesis%20-%20corrected.pdf
- Nwoko CO. Trends in phytoremediation of toxic elemental and organic pollutants. Afr J Biotechnol. 2010;9(37):6010-6016. Available from: https://academicjournals.org/journal/AJB/article-full-text-pdf/894CB7C20320
- Asati A, Pichhode M, Nikhil K. Effect of heavy metals on plants: an overview. Int J Appl Innov Eng Manag. 2016;5(3):56-66. Available from: https://www.researchgate.net/profile/Mohnish-Pichhode/publication/336148391_Effect_of_Heavy_Metals_on_Plants_An_Overview/links/5d92ef25299bf10cff1cd4af/Effect-of-Heavy-Metals-on-Plants-An-Overview.pdf
- Wang W. Literature review on higher plants for toxicity testing. Water Air Soil Pollut. 1991;59:381-400. Available from: https://link.springer.com/article/10.1007/BF00211845
- Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014;7(2):60-72. Available from: https://doi.org/10.2478%2Fintox-2014-0009
- Ivanov V, Zhukovskaya N. Effect of heavy metals on root growth and the use of roots as test objects. Russ J Plant Physiol. 2021;68. Available from: http://dx.doi.org/10.1134/S1021443721070049
- Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012;2012:1-26. Available from: https://doi.org/10.1155/2012/217037
- Sárvári É. Effect of Cd on the iron re-supply-induced formation of chlorophyll-protein complexes in cucumber. Acta Biol Szeged. 2008;52(1):183-186. Available from: https://abs.bibl.u-szeged.hu/index.php/abs/article/view/2618
- Pandhair V, Sekhon BS. Reactive oxygen species and antioxidants in plants: an overview. J Plant Biochem Biotechnol. 2006;15:71-78. Available from: http://dx.doi.org/10.1007/BF03321907
- Huang Z-C, Chen T-B, Lei M, Liu Y-R, Hu T-D. Difference of toxicity and accumulation of methylated and inorganic arsenic in arsenic-hyperaccumulating and-hypertolerant plants. Environ Sci Technol. 2008;42(14):5106-5111. Available from: https://doi.org/10.1021/es703243h
- Abbas G, Murtaza B, Bibi I, Shahid M, Niazi NK, Khan MI, et al. Arsenic uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. Int J Environ Res Public Health. 2018;15(1):59. Available from: https://doi.org/10.3390%2Fijerph15010059
- Shen S, Li X-F, Cullen WR, Weinfeld M, Le X-C. Arsenic binding to proteins. Chem Rev. 2013;113(10):7769-7792. Available from: https://doi.org/10.1021%2Fcr300015c
- Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species generation, hazards, and defense mechanisms in plants under environmental (abiotic and biotic) stress conditions. In: Handbook of Plant and Crop Physiology. CRC Press; 2021; 617-658. Available from: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003093640-37/reactive-oxygen-species-generation-hazards-defense-mechanisms-plants-environmental-abiotic-biotic-stress-conditions-pallavi-sharma-ambuj-bhushan-jha-rama-shanker-dubey-mohammad-pessarakli
- Tiwari AK. Imbalance in antioxidant defence and human diseases: multiple approach of natural antioxidants therapy. Curr Sci. 2001;81(11):1179-1187. Available from: https://www.jstor.org/stable/24106434
- Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R. Abiotic and biotic stress combinations. New Phytol. 2014;203(1):32-43. Available from: https://doi.org/10.1111/nph.12797
- Anjum SA, Tanveer M, Hussain S, Shahzad B, Ashraf U, Fahad S, et al. Osmoregulation and antioxidant production in maize under combined cadmium and arsenic stress. Environ Sci Pollut Res. 2016;23:11864-11875. Available from: https://doi.org/10.1007/s11356-016-6382-1
- Sytar O, Kumar A, Latowski D, Kuczynska P, Strzałka K, Prasad MNV. Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol Plant. 2013;35:985-999. Available from: http://dx.doi.org/10.1007/s11738-012-1169-6
- Anjum NA, Hasanuzzaman M, Hossain MA, Thangavel P, Roychoudhury A, Gill SS, et al. Jacks of metal/metalloid chelation trade in plants—an overview. Front Plant Sci. 2015;6:192. Available from: https://doi.org/10.3389%2Ffpls.2015.00192
- Maiti S, Ghosh N, Mandal C, Das K, Dey N, Adak MK. Responses of the maize plant to chromium stress with reference to antioxidation activity. Braz J Plant Physiol. 2012;24:203-212. Available from: https://www.scielo.br/j/bjpp/a/Q6jyyh7cptjfCVWYBYzqcth/?format=pdf&lang=en