More Information
Submitted: June 18, 2024 | Approved: July 01, 2024 | Published: July 02, 2024
How to cite this article: Kaler AK, Upadhyay SM, Bora NS, Nikam A, Kavya P, et al. Germline BRCA1 Mutation in Squamous Cell Carcinoma of Oesophagus: Driver versus Passenger Mutation. J Genet Med Gene Ther. 2024; 7: 015-019.
DOI: 10.29328/journal.jgmgt.1001011
Copyright License: © 2024 Kaler AK. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Oesophageal cancer; Triple-negative breast cancer; Breast Cancer gene 1
Germline BRCA1 Mutation inSquamous Cell Carcinoma of Oesophagus: Driver versus Passenger Mutation
Amrit Kaur Kaler1*, Shraddha Manoj Upadhyay1, Nandini Shyamali Bora1, Ankita Nikam1, Kavya P1, Nivetha Athikeri2, Dattatray B Solanki2, Imran Shaikh3 and Rajesh Mistry4
1Department of Molecular Pathology and Genomics, Andheri West, Mumbai, India
2Department of Pathology, Andheri West, Mumbai, India
3Department of Gastroenterology, Andheri West, Mumbai, India
4Centre of Cancer, Andheri West, Mumbai, India
*Address for Correspondence: Dr. Amrit Kaur Kaler, Department of Molecular Pathology and Genomics, A902, Adani Western Heights, Opposite Manish Market, Andheri Mumbai, India, Tel: 9886070064; Email: [email protected]; [email protected]
We report a rare case of 62-year-old South Asian women who visited the Molecular Pathology and Genomics Department for hereditary germline cancer genetic testing after being diagnosed with oesophageal cancer, reported as invasive keratinizing squamous cell carcinoma metastasized to the lymph nodes. Her personal history revealed that she was diagnosed with triple-negative breast cancer five years before oesophageal cancer. Germline cancer testing showed pathogenic variants in BRCA1 gene c.68_69delAG, which proved it a hereditary breast and ovarian cancer syndrome. She was started on PARP inhibitors but developed some secondary respiratory failure and succumbed to death.
Less than 10 cases have been reported in the literature of the association of germline BRCA1 and Squamous cell Carcinoma – the esophagus. The article focuses on the probable pathogenesis of BRCA1 mutation with non-classic malignancies and the response of Poly adenosine diphosphate ribose polymerase inhibitors (PARP) inhibitors in such a scenario. We report an unusual manifestation of the BRCA1 gene with second primary oesophageal squamous cell cancer occurring five years later to triple-negative breast cancer.
BRCA1 (BReast CAncer gene 1) or BRCA2 (BReast CAncer gene 2) genes have significantly elevated the risk of developing breast, ovarian, fallopian tubes, pancreatic or prostate cancer, which can either germline or somatic. Breast and Ovarian Cancer Syndrome (HBOC) stands out as one of the prevalent hereditary tumor conditions arising from germline pathogenic variations [1]. BRCA1 pathogenic variations independently serve as adverse prognostic factors, correlating with a heightened risk of cancer recurrence or mortality [2]. A study indicated that out of 1177 cases, 587 cases were positive for the BRCA1 gene; out of which only 11% of the cases were positive for other cancers except breast and ovary which were reported to be present as 60% and 30% respectively [3].
Approximately, 7% of Barrett’s Oesophagus and oesophageal Adenocarcinoma cases are considered familial [4]. The majority of the hereditary cancers in esophageal cancers are BLM/RECQL3, RHBDF2, FANCD1, BRCA2, and FANCN (PALB2) in the literature [5]. However, BRCA 1 familial-based studies showed a high risk of specific cancers tested in the population; the study resulted in an increased relative risk in esophagus cancer of 2.9 (95% CI 1.1-6.0) and stomach at 2.4 (95% CI 1.2-4.3) [6]. Less than 10 cases have been reported in the literature of the association of germline BRCA 1 and Squamous cell Carcinoma – the esophagus [7]. The present case showed BRCA1 pathogenic mutation in association with second primary Squamous Cell Carcinoma (SCC) - esophagus featuring a prior history of Triple-Negative Breast Cancer (TNBC).
A 62 years old female, with a known case of right Triple-Negative Breast Cancer (TNBC), presented with dysphagia, and sore throat for 2 months. Biopsy from the upper esophageal growth for the frozen section showed an invasive keratinizing squamous cell carcinoma. Additional Biopsy from the right cervical lymph node showed tumor cells arranged in diffuse sheets with tumor necrosis, diagnostic of metastatic squamous carcinoma, consistent with an origin from a known primary in the oesophagus. The patient was treated with 3 cycles of chemotherapy with Paclitaxel + Carboplatin but due to complaints of dysphagia chemotherapy planned to switch to Irinotecan+ Cisplatin followed by 12 cycles weekly Paclitaxel.
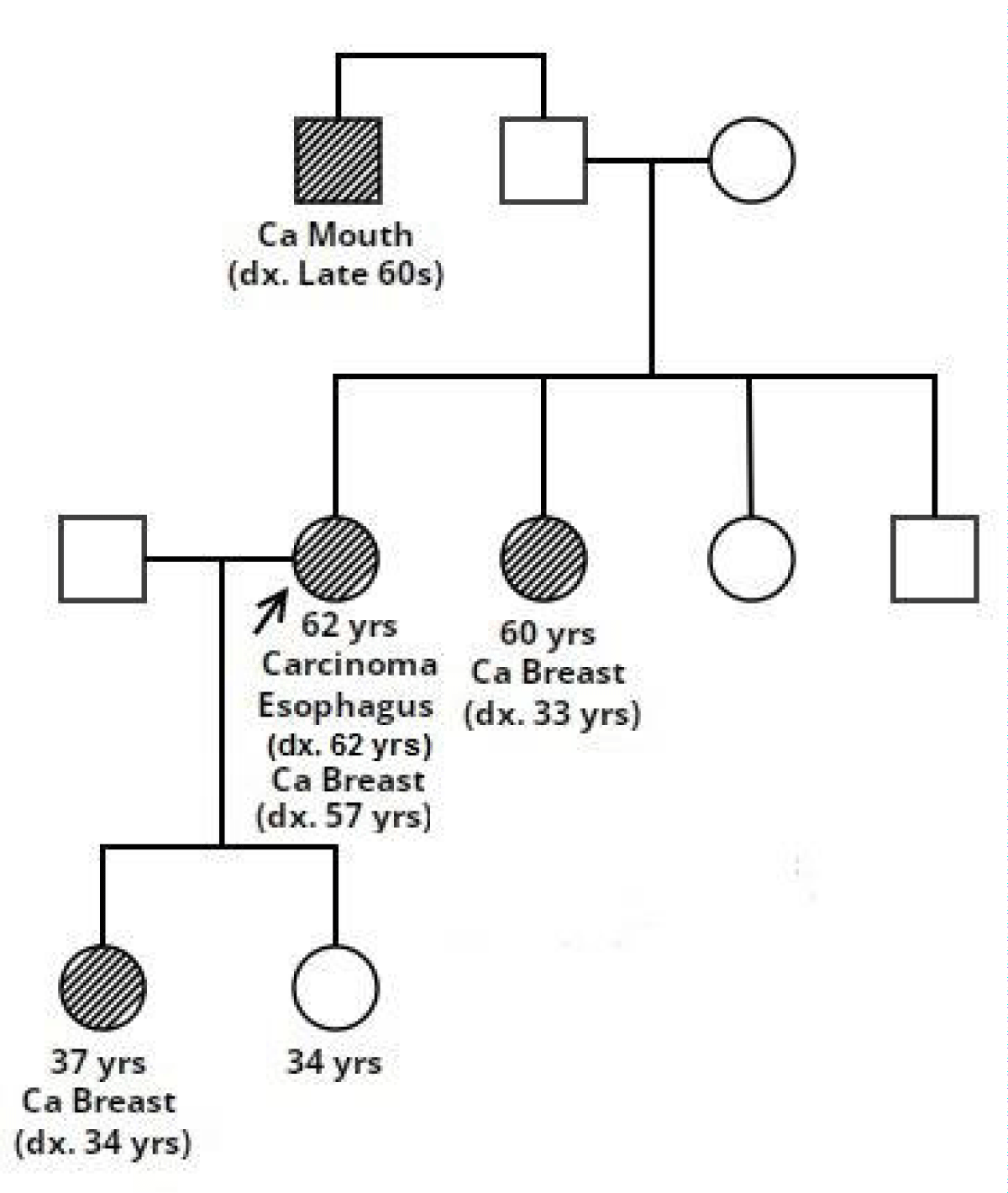
The patient was referred to the Molecular Pathology and Genomics Department for germline testing given dual primary malignancies. The EDTA blood sample was taken and subjected to 113 gene panel hereditary cancer testing using Illumina Dragen enrichment. The reporting was done using ACMG Guidelines. The complete family history was taken and a pedigree chart was drawn. She has a family history of cancer in a sister (60 years) diagnosed at 33 years with breast cancer and a paternal uncle diagnosed in the late 60s with oral cancer. The elder daughter also presented with triple-negative breast cancer at the age of 34 years (Figure 1).
Figure 1: Pedigree of the family which shows multiple affected family members.
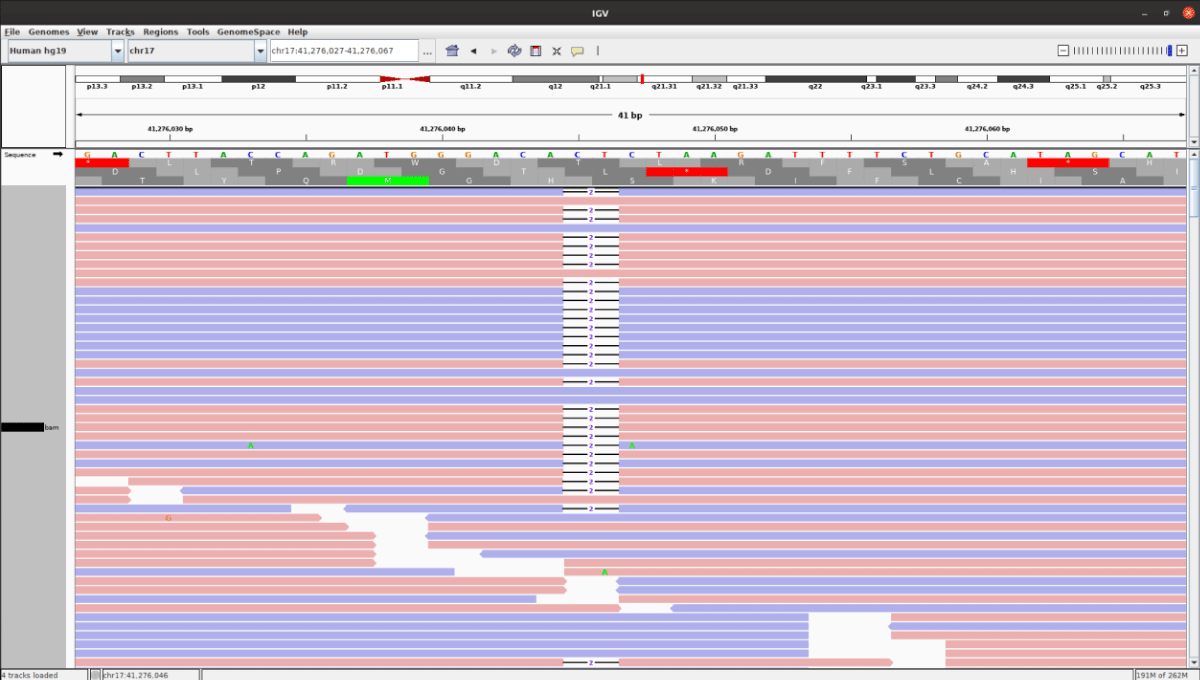
The Bioinformatics analysis detected a pathogenic variant in the BRCA1 gene with a deletion on exon 2 c.68_69delAG (Figure 2). The identified gene mutations are reported based on ACMG guidelines. The mutation confirmation by Sanger sequencing was offered to the family members. Multiplex ligation Probe amplification did not show deletions more than 30bp. The same variant was detected in her elder daughter (37 years) who was diagnosed with TNBC. The patient refused chemotherapy and radiotherapy. Hence, she was counseled and informed about the role of Poly adenosine diphosphate ribose polymerase inhibitors in BRCA1 tumors to which she consented. And then the patient was subsequently started on oral PARP inhibitors but couldn’t recover from secondary respiratory infections and distress.
Figure 2: There was a 2 base pair deletion in the BRCA1 gene (c.68_69delAG) and the sequence alignments were viewed using the Integrative Genomics Viewer (IGV).
BRCA1 (BReast CAncer gene 1) and BRCA2 (BReast CAncer gene 2) are tumor suppressor genes, responsible for encoding proteins that play a crucial role in the repair of damaged DNA via homologous recombination pathway. Individuals carrying harmful variants in either BRCA1 or BRCA2 face elevated risks of gynecological malignancies like the endometrium, fallopian tubes, breast and ovarian cancer, as well as several other types [8,9]. Among the non-classic malignancies, the results of a large-scale registry-based study involving over 63000 patients (data derived from BioBank Japan) revealed that pathogenic variants of BRCA1 and BRCA 2 are linked to an increased risk of esophageal, gastric, and biliary malignancies in addition to the well-known ovarian and breast cancers. Odds ratio [OR]: 17.4 for biliary tract cancer-linked pathogenic variants in BRCA1, and 5.6 for esophageal cancer-linked pathogenic variants in BRCA2. Gastric cancer was linked to variations in BRCA1 (OR, 5.2) and BRCA2 (OR, 4.7) [10]. Conversely, oesophageal cancers with a clinical diagnosis of SCC show a high rate of BRCA2 mutations and have been reported in high-risk regions of northeast India, high- and low-risk Chinese populations, in the Turkmen population of Iran [11-13]. Moran et al, in a family-based study, observed a relative risk increase of Oesophageal cancer (regardless of histology) (relative risk 2.9, 95%CI: 1.1-6) in families with BRCA1 mutations [14]. However, based on a literature search it has been seen that less than 10 cases have been reported of esophageal squamous cell carcinoma linked to a BRCA1 mutation [7]. With this case, we hope to emphasize that PARP inhibitors can potentially be considered as an alternative treatment option.
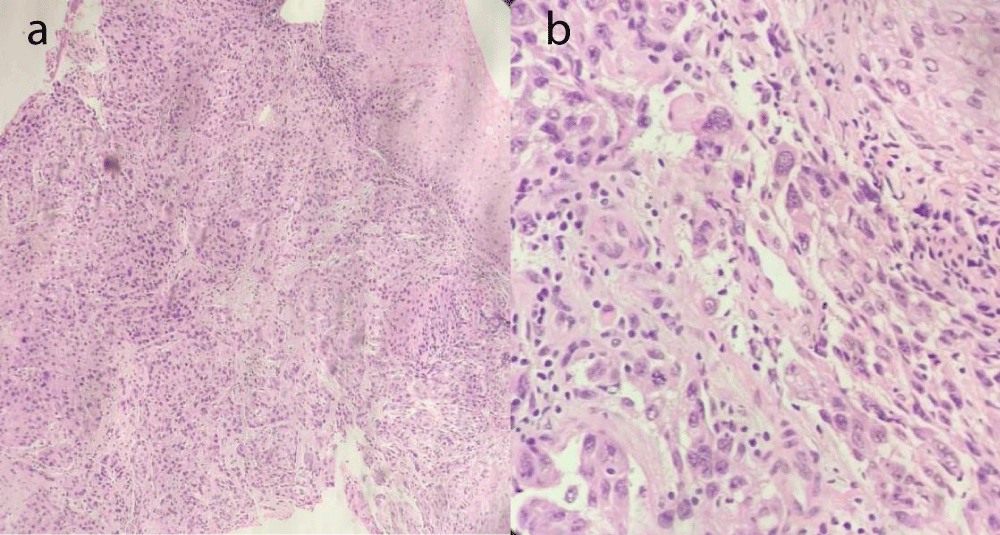
This case centers around a 62-year-old female with a known case of right Triple-Negative Breast Cancer (TNBC). The patient underwent an initial treatment course of 12 cycles of chemotherapy with weekly Paclitaxel, and the patient responded well with disease-free survival for 4 years. Later in 2023, the patient presented with lymphadenopathy. Biopsy from the right cervical lymph node and upper oesophagus revealed an invasive keratinizing squamous carcinoma in the upper esophagus with metastasis to the right cervical lymph node concurrently identified, aligning with the esophageal primary (Figure 3A,B). Tumor cells displayed diffuse sheet arrangements with evidence of necrosis. The patient was started on Paclitaxel+ Carboplatin and then shifted to Irinotecan+ Cisplatin due to the emergence of dysphagia. The patient was referred to the Molecular Pathology and Genomics Department due to dual malignancies for germline cancer testing. Germline cancer testing was performed covering 113 genes panel (Illumina) which revealed a pathogenic variant in the BRCA1 gene (c.68_69delAG), as identified in her elder daughter, diagnosed with TNBC at 34 years old.
Figure 3: A and B: Oesophageal biopsy showing stratified Squamous Carcinoma showed an invasive keratinizing squamous carcinoma showing singly scattered malignant cells arranged in sheets with high N/C ratio, pleomorphism, vesicular nucleus, and scanty cytoplasm.
Approvals of PARPi in classic malignancies
The US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have approved PARP inhibitors, namely olaparib, niraparib, rucaparib, and talazoparib, as monotherapies for the treatment of breast, ovarian, fallopian, primary peritoneal, prostate, and pancreatic cancer [15]. The “synthetic lethality” underlies all of these approvals, to be considered eligible for PARPi therapy is based on HR gene status HR gene activity a HR repair. The HR repair mechanism involves six important HR proteins (BRCA1, BRCA2, ATM, RAD51, MRE11, and PALB2) that predict response to PARP inhibitors and are included in FDA-approved [16]. Because of the alteration in HR proteins repair mechanism is lost which makes cancer cells more vulnerable to PARPi monotherapy [10]. Apart from its potential applications as a stand-alone treatment, PARPi could potentially be beneficial when combined with other therapies which makes it an attractive therapeutic option. Through a variety of pathways, targeting the oncogenic factors PI3K, MEK, and CDK 4/6 decrease HR repair proficiency [17].
PARPi in Gastrointestinal (GI) cancers
Combining PARP inhibitors with chemotherapy and radiation therapy are currently used to treat GI malignancies, may enhance treatment response. Currently, some clinical trials are assessing the effectiveness and safety of this strategy [17]. In clinical studies, the use of PARPi monotherapy for GI tumors is currently being studied. Apart from its function in the development of tumors and the advancement of gastrointestinal malignancies, the DDR PARP enzymes also affect the reaction to treatment [18]. Polyadenosine diphosphate ribose polymerase inhibitors (PARPi) cause synthetic lethality in DNA repair and synthesis [19]. High-fidelity DNA repair is integral to cell survival and repair of DNA [20]. Repair processes are influenced by double-strand breaks (DSB) [21]. In the absence of functional HR, pathways, SBR are converted into DSB which are converted towards error-prone with Non-Homologous End Joining (NHEJ) [22]. The accumulation of DNA errors leads to progressive genomic instability and cell death. This suggests that PARPi may have a function in boosting the effectiveness of cytotoxic drugs that damage DNA. Pre-clinical and clinical research on this targeting strategy is presently being conducted on GI malignancies [17].
Resistance to PARP inhibitors
The patient refused to take systemic chemotherapy and radiation therapy and was offered PARP inhibitor based on Germline testing but progressed on disease 3 months later and finally succumbed to death. There is seen to be a commensurate rise in the number of women with de novo or acquired resistance to PARPi as the drug is used more frequently. A significant percentage of women progress on treatment, as evidenced by numerous trials assessing PARPi as monotherapy or as post-chemotherapy maintenance [17]. This underscores the urgent need to identify the best post-progression treatment, the selection of which may be influenced by the mechanism(s) of PARPi resistance. However, several studies have proven resistance to PARPi owing to alterations in PARP1, restoration of functional HR-associated proteins (BRCA and RAD51 related mutations, demethylation, loss of 53BP1 and DYNLL1, increased histone methylation) and replication fork stability and PARPi efficacy [23].
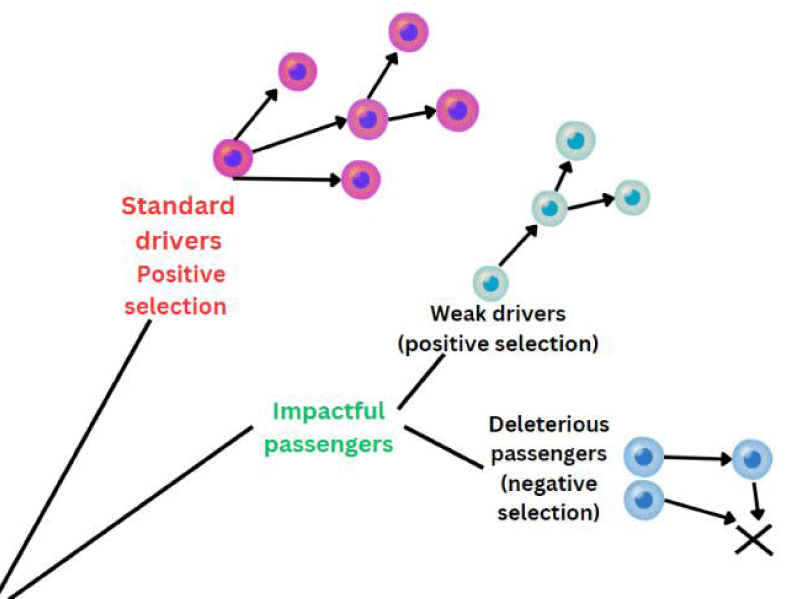
Zabloska et al evaluated the risk of SCC following chest wall radiation in patients with breast cancer and the risk was confined to the upper and middle esophagus [24]. Another possibility could be whether the BRCA 1 mutation in the present scenario is considered the primary driver or passenger mutation (Figure 4). In re-occurrent and multi-sequenced cancers, the majority of mutations are shown to be passengers. The significance of these passenger mutations is largely unknown. However, literature has classified them as effectively neutral and shows a dual weak effect on progression but their cumulative effect can proportionately increase the effect of driver mutations, leading to a tug-of-war between drivers and passengers [25]. Likely, the tumors not classically associated with BRCA1 mutations do not respond as favorably to PARP inhibitors [26].
Figure 4: Cancer cell can acquire both deleterious passenger mutation and impactful drivers. Because of tug of war between drivers and passengers, can lead to either progress or regress. Accumulation of passenger mutations leads to cancer meltdown and slow cancer progression.
Positive preclinical research, however, suggests that PARPi in conjunction with a DNA-damaging agent may be advantageous in this situation [27]. Additionally, Oesophageal Carcinoma cells seem to be more sensitive to fractionated proton irradiation when exposed to the PARPi olaparib [11].
However, phase 1 clinical trials enrolling oesophageal cancers investigating fluzoparib with paclitaxel and apatinib in gastro-oesophageal adenocarcinomas showed a median PFS of 4.9 months and the benefit of this regimen is still unknown [28]. Another combination is the combination of FOLFIRI with Rucaparib is under investigation in oesophageal adenocarcinoma [29].
The identification of a pathogenic BRCA1 variant unveils a hereditary aspect of the patient’s cancer journey. Simultaneous TNBC and esophageal SCC pose intriguing questions about shared genetic factors or underlying mechanisms. An interesting logic in carcinogenesis could be the role of previous radiation in addition to the BRCA 1 mutation. This limitation was the inability to measure the disease response as the patient succumbed to death due to secondary causes. The present case also highlights the need to undergo treatment with PARP inhibitors to understand the significance of the gene and its potential association as a driver or passenger mutation in non-classical malignancies. Probably, the presence of squamous carcinoma in the esophagus and cervical lymph nodes further complicates the clinical picture, emphasizing the necessity for further exploration and genetic counseling for at-risk family members.
- Petrucelli N, Daly MB, Pal T. BRCA1- and BRCA2-Associated Hereditary Breast and Ovarian Cancer. 1998 Sep 4 [updated 2023 Sep 21]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2024. PMID: 20301425.
- Starr J, Ramnaraign B. Germline BRCA1 mutated esophageal squamous cell carcinoma. Rare Tumors. 2020 Nov 5;12:2036361320972218. doi: 10.1177/2036361320972218. PMID: 33224455; PMCID: PMC7649882.
- Mersch J, Jackson MA, Park M, Nebgen D, Peterson SK, Singletary C, Arun BK, Litton JK. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer. 2015 Jan 15;121(2):269-75. doi: 10.1002/cncr.29041. Epub 2014 Sep 15. Erratum in: Cancer. 2015 Jul 15;121(14):2474-5. doi: 10.1002/cncr.29357. PMID: 25224030; PMCID: PMC4293332.
- van Nistelrooij AM, Dinjens WN, Wagner A, Spaander MC, van Lanschot JJ, Wijnhoven BP. Hereditary Factors in Esophageal Adenocarcinoma. Gastrointest Tumors. 2014 Jun;1(2):93-8. doi: 10.1159/000362575. Epub 2014 May 9. PMID: 26675496; PMCID: PMC4645576.
- Esophageal and Esophagogastric Junction Cancers. NCCN Clinical Practice Guidelines in Oncology. Version 3.2023.
- Mersch J, Jackson MA, Park M, Nebgen D, Peterson SK, Singletary C, Arun BK, Litton JK. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer. 2015 Jan 15;121(2):269-75. doi: 10.1002/cncr.29041. Epub 2014 Sep 15. Erratum in: Cancer. 2015 Jul 15;121(14):2474-5. doi: 10.1002/cncr.29357. PMID: 25224030; PMCID: PMC4293332.
- Novikov A, Lourdusamy V, Sheth N, Bedanie G, Balassiano N, Matin M, Walfish A. Bringing Awareness to BRCA1 Associated Esophageal Squamous Cell Carcinoma. Am J Gastroenterol. 2023 Oct;118(10S). doi: 10.14309/01.ajg.0000960380.78519.e.
- Petrucelli N, Daly MB, Pal T. BRCAs1- and BRCA2-Associated Hereditary Breast and Ovarian Cancer. In: Adam MP, Feldman J, Mirzaa GM, et al. editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2023. https://www.ncbi.nlm.nih.gov/books/NBK1247/. Updated 2023 Sep 21.
- Levy-Lahad E, Friedman E. Cancer risks among BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2007 Jan 15;96(1):11-15. doi: 10.1038/sj.bjc.6603535.
- Momozawa Y, Sasai R, Usui Y, Shiraishi K, Iwasaki Y, Taniyama Y, Parsons MT, Mizukami K, Sekine Y, Hirata M, Kamatani Y, Endo M, Inai C, Takata S, Ito H, Kohno T, Matsuda K, Nakamura S, Sugano K, Yoshida T, Nakagawa H, Matsuo K, Murakami Y, Spurdle AB, Kubo M. Expansion of Cancer Risk Profile for BRCA1 and BRCA2 Pathogenic Variants. JAMA Oncol. 2022 Jun 1;8(6):871-878. doi: 10.1001/jamaoncol.2022.0476. PMID: 35420638; PMCID: PMC9011177.
- Maccaroni E, Giampieri R, Lenci E, Scortichini L, Bianchi F, Belvederesi L, Brugiati C, Pagliaretta S, Ambrosini E, Berardi R. BRCA mutations and gastrointestinal cancers: When to expect the unexpected? World J Clin Oncol. 2021 Jul 24;12(7):565-580. doi: 10.5306/wjco.v12.i7.565. PMID: 34367929; PMCID: PMC8317649.
- Hu N, Wang C, Han XY, He LJ, Tang ZZ, Giffen C, Emmert-Buck MR, Goldstein AM, Taylor PR. Evaluation of BRCA2 in the genetic susceptibility of familial esophageal cancer. Oncogene. 2004 Jan 22;23(3):852-8. doi: 10.1038/sj.onc.1207150. PMID: 14647438.
- Akbari MR, Malekzadeh R, Nasrollahzadeh D, Amanian D, Islami F, Li S, Zandvakili I, Shakeri R, Sotoudeh M, Aghcheli K, Salahi R, Pourshams A, Semnani S, Boffetta P, Dawsey SM, Ghadirian P, Narod SA. Germline BRCA2 mutations and the risk of esophageal squamous cell carcinoma. Oncogene. 2008 Feb 21;27(9):1290-6. doi: 10.1038/sj.onc.1210739. Epub 2007 Aug 27. PMID: 17724471.
- Moran A, O'Hara C, Khan S, Shack L, Woodward E, Maher ER, Lalloo F, Evans DG. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam Cancer. 2012 Jun;11(2):235-42. doi: 10.1007/s10689-011-9506-2. PMID: 22187320.
- Revythis A, Limbu A, Mikropoulos C, Ghose A, Sanchez E, Sheriff M, Boussios S. Recent Insights into PARP and Immuno-Checkpoint Inhibitors in Epithelial Ovarian Cancer. Int J Environ Res Public Health. 2022 Jul 14;19(14):8577. doi: 10.3390/ijerph19148577. PMID: 35886427; PMCID: PMC9317199.
- Patel PS, Algouneh A, Hakem R. Exploiting synthetic lethality to target BRCA1/2-deficient tumors: where we stand. Oncogene. 2021 Apr;40(17):3001-3014. doi: 10.1038/s41388-021-01744-2. Epub 2021 Mar 14. PMID: 33716297.
- Alhusaini A, Cannon A, Maher SG, Reynolds JV, Lynam-Lennon N. Therapeutic Potential of PARP Inhibitors in the Treatment of Gastrointestinal Cancers. Biomedicines. 2021 Aug 16;9(8):1024. doi: 10.3390/biomedicines9081024. PMID: 34440228; PMCID: PMC8392860.
- Boehler C, Gauthier LR, Mortusewicz O, Biard DS, Saliou JM, Bresson A, Sanglier-Cianferani S, Smith S, Schreiber V, Boussin F, Dantzer F. Poly(ADP-ribose) polymerase 3 (PARP3), a newcomer in cellular response to DNA damage and mitotic progression. Proc Natl Acad Sci U S A. 2011 Feb 15;108(7):2783-8. doi: 10.1073/pnas.1016574108. Epub 2011 Jan 26. PMID: 21270334; PMCID: PMC3041075.
- Ashworth A, Lord CJ, Reis-Filho JS. Genetic interactions in cancer progression and treatment. Cell. 2011 Apr 1;145(1):30-8. doi: 10.1016/j.cell.2011.03.020. PMID: 21458666.
- Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, Ashworth A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005 Apr 14;434(7035):917-21. doi: 10.1038/nature03445. PMID: 15829967.
- Torgovnick A, Schumacher B. DNA repair mechanisms in cancer development and therapy. Front Genet. 2015 Apr 23;6:157. doi: 10.3389/fgene.2015.00157. PMID: 25954303; PMCID: PMC4407582.
- Beck C, Boehler C, Guirouilh Barbat J, Bonnet ME, Illuzzi G, Ronde P, Gauthier LR, Magroun N, Rajendran A, Lopez BS, Scully R, Boussin FD, Schreiber V, Dantzer F. PARP3 affects the relative contribution of homologous recombination and nonhomologous end-joining pathways. Nucleic Acids Res. 2014 May;42(9):5616-32. doi: 10.1093/nar/gku174. Epub 2014 Mar 5. PMID: 24598253; PMCID: PMC4027158.
- Lee EK, Matulonis UA. PARP inhibitor resistance mechanisms and implications for post-progression combination therapies. Cancers. 2020 Jul 25;12(8):2054. https://doi.org/10.3390/cancers12082054.
- Zablotska LB, Chak A, Das A, Neugut AI. Increased risk of squamous cell esophageal cancer after adjuvant radiation therapy for primary breast cancer. Am J Epidemiol. 2005 Feb 15;161(4):330-7. doi: 10.1093/aje/kwi050. PMID: 15692076.
- McFarland CD, Yaglom JA, Wojtkowiak JW, Scott JG, Morse DL, Sherman MY, Mirny LA. The Damaging Effect of Passenger Mutations on Cancer Progression. Cancer Res. 2017 Sep 15;77(18):4763-4772. doi: 10.1158/0008-5472.CAN-15-3283-T. Epub 2017 May 23. PMID: 28536279; PMCID: PMC5639691.
- Sokol ES, Jin DX, Fine A, Trabucco SE, Maund S, Frampton G, Molinero L, Antonarakis ES. PARP Inhibitor Insensitivity to BRCA1/2 Monoallelic Mutations in Microsatellite Instability-High Cancers. JCO Precis Oncol. 2022 Jun;6:e2100531. doi: 10.1200/PO.21.00531. PMID: 35772050; PMCID: PMC9259120.
- Kitahara M, Hozumi Y, Machinaga M, Hayashi Y. A Case of Triple-Negative Breast Cancer with Germline Pathogenic Variants in Both BRCA1 and BRCA2. Case Rep Oncol. 2021 Nov 18;14(3):1645-1651. doi: 10.1159/000520148. PMID: 35082620; PMCID: PMC8740154.
- Xu JM, Liu R, Ba Y, Jiang D, Wang M, Zheng Y, Wei J, Bai YX, Lin L, Xiong J, et al. Phase I study of fluzoparib, a PARP1 inhibitor in combination with apatinib and paclitaxel in patients with advanced gastric and gastroesophageal junction adenocarcinoma. J Clin Oncol. 2019;37(Suppl S15):4060. doi: 10.1200/JCO.2019.37.15_suppl.4060.
- Bekaii-Saab TS. Phase I study of irinotecan liposome (Nal-IRI), fluorouracil, leucovorin and rucaparib in the treatment of select gastrointestinal metastatic malignancies followed by a phase Ib of first and second line treatment of both unselected and selected (for BRCA 1/2 and PALB2 mutations) patients with metastatic adenocarcinoma of the pancreas then followed by a phase II study of first-line treatment of selected patients with metastatic adenocarcinoma of the pancreas with genomic markers (signature) of homologous recombination deficiency (HRD). In: Liposomal irinotecan, fluorouracil, leucovorin calcium, and rucaparib in treating patients with metastatic pancreatic, colorectal, gastroesophageal, or biliary cancer. Mayo Clinic: Arizona, FL, USA; 2017.